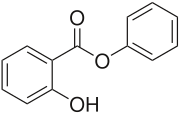
Phenyl salicylate
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenyl 2-hydroxybenzoate | |
| Other names
Salol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.873 |
| EC Number |
|
| KEGG | |
| MeSH | C026041 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H10O3 | |
| Molar mass | 214.22 g/mol |
| Appearance | White solid |
| Density | 1.25 g/cm3 |
| Melting point | 41.5 °C (106.7 °F; 314.6 K) |
| Boiling point | 173 °C (343 °F; 446 K) at 12 mmHg |
| 1 g/6670 mL | |
| -123.2·10−6 cm3/mol | |
Refractive index (nD)
|
1.615[2] |
| Pharmacology | |
| G04BX12 (WHO) | |
| Hazards | |
| Flash point | 137.3[2] °C (279.1 °F; 410.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenyl salicylate, or salol, is the organic compound with the formula C6H5O2C6H4OH. It is a white solid. It is occasionally used in sunscreens and as an antiseptic.[3]
Production and reactions
The title compound was synthesized first in 1883 by the Polish chemist and doctor Marceli Nencki (who didn't publish his findings) and then independently in 1885 by the German chemist Richard Seifert (de) (1861–1919) (who did publish his findings). It is synthesized by heating salicylic acid with phenol in the presence of phosphoryl chloride.[4] It also arises from heating salicylic acid:[5]
- 2 HOC6H4CO2H → C6H5O2C6H4OH + CO2 + H2O
The conversion entails dehydration and decarboxylation. Heating phenyl salicylate in turn gives xanthone.[6][3]
- 2 C6H5O2C6H4OH → 2 C6H5OH + O[C6H4]2CO + CO2
In this conversion, phenol is produced as well as carbon dioxide.
Salol reaction
In the salol reaction, phenyl salicylate reacts with o-toluidine in 1,2,4-trichlorobenzene at elevated temperatures to the corresponding amide o-salicylotoluide.[7] Salicylamides are a type of drug.
Medical
It has been used as an antiseptic[8] based on the antibacterial activity upon hydrolysis in the small intestine.[citation needed]
It acts as a mild analgesic.[9]
History
The Swiss physician Hermann Sahli (sometimes spelled "Saly") (1856–1933) sought a substitute for sodium salicylate, which was used as a treatment for rheumatoid arthritis but which wasn't tolerated by some patients. So Dr. Sahli asked the Polish chemist and doctor Marceli Nencki of Bern, Switzerland, if he knew of a salicylate compound that lacked sodium salicylate's side effects.[10] Nencki recommended phenyl salicylate, which he had synthesized circa 1883.[11][12] While Nencki had been investigating how phenyl salicylate behaved in the body, he hadn't published his findings.[13] Meanwhile, the German chemist Richard Seifert (de) (1861–1919), a student of the German chemist Rudolf Wilhelm Schmitt (de) (1830–1898),[14] independently synthesized phenyl salicylate in 1885.[15] In 1885, Seifert accepted a position at the Heyden chemical corporation (de) of Radebeul, Germany, which manufactured salicylic acid.[16] The United States granted to Nencki and Seifert a patent for the production of phenyl salicylate,[17] whereas Germany granted a patent for its production to Nencki and the Heyden corporation.[18] The Heyden company subsequently sold phenyl salicylate as a pharmaceutical, under the commercial name "Salol",[19] a contraction of "SALicylate of phenOL".[20] Among other applications,[21] Salol was used as an orally administered antiseptic for the small intestine, where the compound is hydrolyzed into salicylic acid and phenol.[17]
See also
Phenyl salicylate is used in school laboratory demonstrations on how cooling rates affect crystal size in igneous rocks, and can be used to demonstrate seed crystal selectiveness.
References
- ^ Merck Index, 11th Edition, 7282.
- ^ a b ChemBK Chemical Database http://www.chembk.com/en/chem/Phenyl%20salicylate
- ^ a b Boullard, Olivier; Leblanc, Henri; Besson, Bernard (2000). "Salicylic Acid". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a23_477. ISBN 3527306730.
- ^ Gaylord, Norman G.; Kamath, P. M. (1952). "p-Chlorophenyl Salicylate". Organic Syntheses. 32: 25. doi:10.15227/orgsyn.032.0025.
- ^ Kuriakose, G.; Nagaraju, N. (2004). "Selective Synthesis of Phenyl Salicylate (Salol) by Esterification Reaction over Solid Acid Catalysts". Journal of Molecular Catalysis A: Chemical. 223 (1–2): 155–159. doi:10.1016/j.molcata.2004.03.057.
- ^ A. F. Holleman (1927). "Xanthone". Org. Synth. 7: 84. doi:10.15227/orgsyn.007.0084.
- ^ Allen, C. F. H.; VanAllan, J. (1946). "SALICYL-o-TOLUIDE" (PDF). Organic Syntheses. 26: 92; Collected Volumes, vol. 3, p. 765.
- ^ Walter Sneader (2005). Drug discovery: a history. John Wiley and Sons. pp. 358–. ISBN 978-0-471-89980-8. Retrieved 28 October 2010.
- ^ Judith Barberio (4 September 2009). Nurse's Pocket Drug Guide, 2010. McGraw Hill Professional. pp. 57–. ISBN 978-0-07-162743-6. Retrieved 28 October 2010.
- ^ See:
- (Staff) (29 April 1886). "Ueber des Salol" [On Salol]. Wiener Medizinische Blätter (in German). 9 (17): 524. From column 524: "Das salicylsaure Natron ist zwar das verzüglichste Specificum … Dieser Körper, welchen N. "Salol" nannte, … " (Sodium salicylate is indeed the best remedy against acute rheumatoid arthritis; however, as is known, it's poorly tolerated by some patients who, after its use, suffer nausea, tinnitus, etc., occasionally even fainting spells. These inconveniences caused Dr. Sahli (La Semaine Médicale [The Medical Week], no. 15, 14 April 1886 ; Allgemeine medizinische Central-Zeitung [General Medical Central Journal]) to inquire of Dr. Nencki whether he knew another salicylate compound that didn't have those drawbacks. Soon thereafter he received from Nencki a communication that he had succeeded in producing a compound in which an ether (phenol) had been substituted [for one of salicylic acid's hydrogen atoms], instead of a base [i.e., metal alkali]. This substance, which Nencki named "Salol", … )
- Reprinted in: (Staff) (1886). "Salol". Pharmazeutische Zentralhalle für Deutschland (in German). 27: 219.
- Boymond (1886). "Sur le salol" [On salol]. Bulletin Général de Thérapeutique Médicale et Chirurgicale (in French). 111: 122–124. From pp.122–123: "Dans une communication faite à la Société de médecine et de pharmacie de Berne, le docteur Sahli a fait ressortir les effets funestes produits sur l'estomac par un usage prolongé du salicylate de soude. Pour parer à cet inconvénient, il s'est adressé au professeur de Nencki, pour lui demander s'il n'y aurait pas possibilité de trouver une autre combinaison de l'acide salicylique. Le professeur de Nencki ayant étudié, il y a trois ans, les propriétés chimiques, physiologiques et surtout antiseptiques de salicylate de phénol ou salol, conseilla immédiatement ce produit au docteur Sahli." (In a communication [that he] made to the Berne Society of Medicine and Pharmacy, Dr. Sahli highlighted the harmful effects [that are] produced on the stomach by the prolonged use of sodium salicylate. In order to deal with this drawback, he spoke to Prof. Nencki, in order to ask him if there wasn't a possibility of finding another compound of salicylic acid. Prof. Nencki, having studied, three years ago, the chemical, physiological, and above all antiseptic properties of phenyl salicylate or salol, immediately recommended this product to Dr. Sahli.)
- ^ Sahli, Hermann (1886). "Ueber die therapeutische Anwendung des Salols (des salicylsauren Phenoläthers)" [On the therapeutic application of Salol (of the ester phenyl salicylate)]. Correspondenz-Blatt für schweizer Aerzte (Correspondence Journal for Swiss Doctors) (in German). 16: 321–325. This is the speech which Sahli made to the Berne Society of Medicine and Pharmacy, and in which he announced Salol and its use as a new pharmaceutical. From p. 321: "Diese Verbindung war neben mehreren verwandten zusammengesetzten Aethern von Phenolen und org. Säuren von Nencki schon vor 3 Jahren dargestellt und auf seine antiseptischen und physiologisch-chemischen Eigenschaften untersucht worden, hat aber eine practische Anwendung bisher noch nicht gefunden." (In addition to several related esters composed of phenol and organic acids, this compound was prepared 3 years ago by Nencki and was investigated for its antiseptic and physiological-chemical properties; however, [he] hadn't yet found a practical application.)
- ^ Levinstein, Ivan (January 1, 1887). "Notes on a few chemical substances recently introduced into the field of chemical industry". The Pharmaceutical Journal and Transactions. 3rd series. 17: 527–530. From p. 527: "Salol was produced about three years ago by Professor Nencki, who also investigated its physiological properties."
- ^ However, in 1885, Nencki did publish his findings about the hydrolysis, by the body, of esters. In particular, Nencki published his findings regarding the hydrolysis of phenyl benzoate (Phenolbenzoesäureester), a compound that is chemically closely related to phenyl salicylate. See pp. 380-382 of: Nencki (1885). "Ueber die Spaltung der Säureester der Fettreihe und der aromatischen Verbindungen im Organismus und durch das Pankreas" [On the hydrolysis of esters of acids of the aliphatic series and of aromatic compounds in vivo and by the pancreas]. Archiv für experimentelle Pathologie und Pharmakologie (in German). 20: 377–384.
- ^ Sorms, Bernhard (2007). "Schmitt, Rudolf". Neue Deutsche Biographie (in German). Vol. 23. Berlin, Germany: Duncker & Humblot. pp. 241–242. ISBN 978-3-428-11204-3. From p. 241: "Sein bekanntester Schüler war Richard Seifert (1861-1919), der Chefchemiker der Fabrik v. Heydens, der u.a. 1892 die Rezeptur für das weltbekannte Mundwasser "Odol" entwickelte." (His [Schmitt's] best known pupil was Richard Seifert (1861-1919), the chief chemist of the Heyden factory, who, among others, developed in 1892 the formula for the world-famous mouthwash "Odol".)
- ^ See:
- Seifert, Richard (1885). "Ueber die Einwirkung von Natriummerkaptid auf Phenylester" [On the action of sodium mercaptide (sodium ethanethiolate, C2H5SNa) on phenyl esters]. Journal für Praktische Chemie. 2nd series (in German). 31: 462–480. doi:10.1002/prac.18850310138.; see: "VII. Einwirkung von Natriummerkaptid auf Phenylsalicylat und Phenylmethylsalicylat. Thioäthylmethylsalicylat." (VII. Action of sodium mercaptide on phenylsalicylate and phenylmethylsalicylate. Thioethylmethylsalicylate.), p. 472. From p. 472: "Zur Darstellung von Phenylsalicylat … Phosphoroxychlorid (etwas über 1/3 Molekül) hinzu." (For the preparation of phenyl salicylate, I melted 69 grams of salicylic acid (1 mole) with 48 grams of phenol (1 mole) at about 135°C and added, in small portions, 28 grams of phosphoryl chloride (somewhat over 1/3 mole).) On p. 473, Seifert said that phenyl salicylate had not been synthesized previously: "Das Phenylsalicylat ist noch nicht dargestellt worden." (Phenyl salicylate has not been prepared until now.)
- English abstract: Seifert, R. (1885). "Action of sodium mercaptide on phenyl salts". Journal of the Chemical Society. 48 (1): 1057–1058.
- ^ Arevipharma, Geschichter der chemischen Fabrik von Heyden: Vergrösserung der Fabrik und Aufnahme wichtger neuer Präparate [History of the Heyden chemical factory: Expansion of the factory and addition of important new preparations]
- ^ a b Nencki, Marrel V. ; Seifert, Richard "Production of salol" U.S. Patent no. 350,012 (filed: 22 July 1886 ; issued: 28 September 1886).
- ^ (Staff) (1886). "D.R.P. no. 38973 Kl. 22. M. Nencki in Bern und Dr. Fr. v. Heyden Nachfolger in Radebeul bei Dresden. Verfahren zur Darstellung der Salicylssäureester der Phenole und Naphtole, genannt "Salole"" [German Reich Patent no. 38973 Class 22. M. Nencki in Bern and Dr. Friedrich von Heyden's successor in Radebeul near Dresden. Procedure for the preparation of the salicylic acid ester of phenol and naphthol, called "Salol"]. Fortschritte der Teerfarbenfabrikation und Verwandter Industriezweige (Progress in the Fabrication of Coal Tar Dyes and Related Branches of Industry) (in German). 1: 237–239.
- ^ See:
- Arevipharma, Geschichter der chemischen Fabrik von Heyden: Vergrösserung der Fabrik und Aufnahme wichtger neuer Präparate [History of the Heyden chemical factory: Expansion of the factory and addition of important new preparations]
- Schlenk, O. (1934). Chemische Fabrik von Heyden, Aktiengesellschaft, Radebeul-Dresden, 1874-1934. Erinnerungsblatter aus 6 Jahrzehnten [Chemical Factory of Heyden, Inc., Radebeul-Dresden, 1874-1934. Recollections from 6 Decades] (in German). Radebeul, Germany: Kupky & Dietze.
- ^ Moss, John (2 October 1886). "Salol: a new antiseptic". The Pharmaceutical Journal and Transactions. 3rd series. 17: 273–274. From p. 273: " … the word salol is evidently compounded of the initial and terminal letters of the former title [i.e., salicylate of phenol]"
- Reprinted in: Moss, John (1886). "Salol: a new antiseptic". American Journal of Pharmacy. 58: 552–555.
- ^ (Heyden corporation) (1887). Salol, the New Remedy for Rheumatism and Rheumatic Affections. New York City, New York, USA: W.H. Schieffelin & Co.
