Pterygium (eye)
| Pterygium (eye) | |
|---|---|
| Other names | surfer's eye[1] |
| Specialty | Ophthalmology |
Pterygium most often refers to a benign growth of the conjunctiva. A pterygium commonly grows from the nasal side of the conjunctiva. It is usually present in the palpebral fissure. It is associated with and thought to be caused by ultraviolet-light exposure (e.g., sunlight), low humidity, and dust. The predominance of pterygis on the nasal side is possibly a result of the sun's rays passing laterally through the cornea, where it undergoes refraction and becomes focused on the limbic area. Sunlight passes unobstructed from the lateral side of the eye, focusing on the medial limbus after passing through the cornea. On the contralateral (medial) side, however, the shadow of the nose medially reduces the intensity of sunlight focused on the lateral/temporal limbus.[2]
Signs and symptoms
Symptoms of pterygium include persistent redness from smoke,[2] inflammation,[3] foreign body sensation, tearing, dry and itchy eyes. In advanced cases the pterygium can affect vision[3] as it invades the cornea with the potential of obscuring the optical center of the cornea and inducing astigmatism and corneal scarring.[4][5] Many patients do complain of the cosmetic appearance of the eye either with some of the symptoms above or as their major complaint.
Pathology

Pterygium in the conjunctiva is characterized by elastotic degeneration of collagen (actinic elastosis[6]) and fibrovascular proliferation. It has an advancing portion called the head of the pterygium, which is connected to the main body of the pterygium by the neck. Sometimes a line of iron deposition can be seen adjacent to the head of the pterygium called Stocker's line. The location of the line can give an indication of the pattern of growth.
The exact cause is unknown, but it is associated with excessive exposure to wind, sunlight, or sand. Therefore, it is more likely to occur in populations that inhabit the areas near the equator, as well as windy locations. In addition, pterygia are twice as likely to occur in men than women. Some research also suggests a genetic predisposition due to an expression of vimentin, which indicates cellular migration by the keratoblasts embryological development, which are the cells that give rise to the layers of the cornea. Supporting this fact is the congenital pterygium, in which pterygium is seen in infants.[7] These cells also exhibit an increased P53 expression likely due to a deficit in the tumor suppressor gene. These indications give the impression of a migrating limbus because the cellular origin of the pterygium is actually initiated by the limbal epithelium.[8]
The pterygium is composed of several segments:
- Fuchs' Patches (minute gray blemishes that disperse near the pterygium head)
- Stocker's Line (a brownish line composed of iron deposits)
- Hood (fibrous nonvascular portion of the pterygium)
- Head (apex of the pterygium, typically raised and highly vascular)
- Body (fleshy elevated portion congested with tortuous vessels)
- Superior Edge (upper edge of the triangular or wing-shaped portion of the pterygium)
- Inferior Edge (lower edge of the triangular or wing-shaped portion of the pterygium).
Diagnosis
Pterygium (conjunctiva) can be diagnosed without need for a specific exam, however corneal topography is a practical test (technique) as the condition worsens.[9][10]
Prevention
As it is associated with excessive sun [11] or wind exposure, wearing protective sunglasses with side shields and/or wide brimmed hats and using artificial tears throughout the day may help prevent their formation or stop further growth. Surfers and other water-sport athletes should wear eye protection that blocks 100% of the UV rays from the water, as is often used by snow-sport athletes. Many of those who are at greatest risk of pterygium from work or play sun exposure do not understand the importance of protection.[12][13]
Treatment
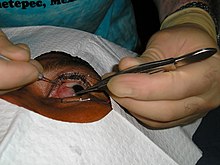
Historically a variety of options are available for the management of pterygium, from irradiation, to conjunctival auto-grafting or amniotic membrane transplantation, along with glue and suture application. Treatments and techniques have evolved and improved due to research and a deeper understanding of pterygium, resulting in some treatments being discontinued.
As it is a benign growth, pterygium typically does not require surgery until it grows to such an extent that it causes visual loss or presents with acute symptoms, or pulls on the cornea, distorting vision. Some of the irritating symptoms can be addressed with artificial tears. In some patients, cosmetic or esthetic concerns may be sufficient to warrant surgical removal. However, no reliable medical treatment exists to reduce or even prevent pterygium progression. Definitive treatment is achieved only by surgical removal. Follow-up of at least a year after surgery is required to identify 97% of recurrences that will occur.
The below treatments are listed in order of effectiveness:
Auto-grafting
Conjunctival auto-grafting is a surgical technique that is an effective and safe procedure for pterygium removal.[medical citation needed] When the pterygium is removed, the tissue that covers the sclera known as the Tenons layer is also removed. Auto-grafting covers the bare sclera with conjunctival tissue that is surgically removed from an area of healthy conjunctiva. That “self-tissue” is then transplanted to the bare sclera and is fixated using sutures or tissue adhesive. One technique, Pterygium Extended Removal Followed by Extended Conjunctival Transplant (P.E.R.F.E.C.T for Pterygium) reduces the risk of recurrence in a first time removal to 1 in 1000,
Amniotic membrane transplantation
Amniotic membrane transplantation is an effective and safe procedure for pterygium removal. Amniotic membrane transplantation offers practical alternative to conjunctival auto graft transplantation for extensive pterygium removal. Amniotic membrane transplantation is tissue that is acquired from the innermost layer of the human placenta and has been used to replace and heal damaged mucosal surfaces including successful reconstruction of the ocular surface. It has been used as a surgical material since the 1940s, and has been shown to have a strong anti-adhesive effect.[14][15] Using an amniotic graft facilitates epithelialization, and has anti-inflammatory as well as surface rejuvenation properties. Amniotic membrane transplantation can also be fixated to the sclera using sutures, or glue adhesive.[16][17][18]
Amniotic membrane transplantation with Tisseel glue application and mitomycin C has shown excellent cosmetic outcomes with a surface free of redness, stitching, or patches, which makes the ocular surface suitable for vision correction surgery sooner.[19] [20] [21] Amniotic membrane by itself does not provide an acceptable recurrence rate.[22]
Mitomycin C may be used with surgery appears to reduce the risk of the problem returning but is associated with side effects.[23]
Research
A Cochrane Review sought to compare the safety and effectiveness of conjunctival autograft surgery against amniotic membrane transplant, in pterygium surgery. In a sample of 1947 eyes from 20 studies, patients who underwent conjunctival autograft surgery were significantly less likely to have a reoccurrence of pterygium compared to patients who underwent amniotic membrane transplant, 6 months after surgery.[24] More research is needed to determine which type of surgery resulted in better vision and quality of life outcomes.[24]
See also
References
- ^ Tollefsbol, Trygve (2016). Medical Epigenetics. Academic Press. p. 395. ISBN 9780128032404.
- ^ a b Coroneo, MT (November 1993). "Pterygium as an early indicator of ultraviolet insolation: a hypothesis". Br J Ophthalmol. 77 (11): 734–9. doi:10.1136/bjo.77.11.734. PMC 504636. PMID 8280691.
- ^ a b Kunimoto, Derek; Kunal Kanitkar; Mary Makar (2004). The Wills eye manual: office and emergency room diagnosis and treatment of eye disease (4th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 50–51. ISBN 978-0781742078.
- ^ Gulani, A.C. (24 March 2005). "Extended Sun Exposure Increases Risk of Eye Pterygium".
- ^ Fisher, J.P.; Trattler, W.B. (12 January 2009). "Pterygium".
- ^ Klintworth, G; Cummings, T. "24; The eye and ocular adnexa". In Stacey, Mills (ed.). Sternberg's Diagnostic Surgical Pathology (5th ed.). ISBN 978-0-7817-7942-5.
- ^ http://www.paramountbooks.com.pk/LoginIndex.asp?title=Concise-Ophthalmology-(pb)-2014&Isbn=9789696370017&opt=3&sUBcAT=06
- ^ Gulani, A; Dastur, YK (January–March 1995). "Simultaneous pterygium and cataract surgery". Journal of postgraduate medicine. 41 (1): 8–11. PMID 10740692. Retrieved 30 November 2012.
- ^ "Pterygium: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 15 August 2016.
- ^ "Pterygium Workup: Imaging Studies, Procedures". emedicine.medscape.com. Retrieved 15 August 2016.
- ^ Mackenzie, F.D.; Hirst, L.W.; Battistutta D.; Green, A.: Risk Analysis in the Development of Pterygia. Ophthalmology. 99(7): 1056-1061, 1992.
- ^ Lee, G.A.; Hirst, L.W.; Sheehan, M.: Knowledge of Sunlight Effects on the Eyes and Protective Behaviours in the General Community. Ophthalmic Epidemiology. Ophthalmic Epidemiology. 1(2):67-84, 1994.
- ^ Lee, G.; Hirst, L.W.; Sheehan, M.: Knowledge of Sunlight effects on the eyes and protective behaviors in adolescents. Ophthalmic Epidemiology. 6(3): 171–180, 1999.
- ^ Trelford, JD; Trelford-Sauder, M (1 August 1979). "The amnion in surgery, past and present". American Journal of Obstetrics and Gynecology. 134 (7): 833–45. PMID 380345.
- ^ Tayyar, M; Turan, R; Ayata, D (June 1993). "The use of amniotic membrane plus heparin to prevent postoperative adhesions in the rabbit". The Tokai journal of experimental and clinical medicine. 18 (1–2): 57–60. PMID 7940608.
- ^ Gulani AC (2007). "Corneoplastique". Techniques in Ophthalmology. 5 (1): 11–20. doi:10.1097/ito.0b013e318036ae0d.
- ^ Gulani AC. "Corneoplastique", Video Journal of Cataract and Refractive Surgery. Volume XXII. Issue 3, 2006.
- ^ Gulani AC (2006). "A New Concept for Refractive Surgery". Ophthalmology Management. 10 (4): 57–63.
- ^ Gulani AC. Vision Corrective Surgeries: Past Techniques, Present Trends and Future Technologies, North East Florida Medicine. 2007; 2 (58) 41-44.
- ^ Gulani AC, Holladay J, Belin M, Ahmed I. Future Technologies in LASIK- Pentacam Advanced Diagnostic for Laser Vision Surgery. In Experts Review of Ophthalmology, 2008- London
- ^ Gulani AC. " Corneoplastique: Art of Laser Vision Surgery"- Corneal Refractive Surgery in Video Atlas of Ophthalmic Surgery. XXXVIII. (2) 2008
- ^ Ophthalmology. 1997 Jun;104(6):974-85.Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Prabhasawat P1, Barton K, Burkett G, Tseng SC
- ^ Martins, TG; Costa, AL; Alves, MR; Chammas, R; Schor, P (2016). "Mitomycin C in pterygium treatment". International journal of ophthalmology. 9 (3): 465–8. PMID 27158622.
- ^ a b Clearfield E, Muthappan V, Wang X, Kuo IC (2016). "Cojunctival autograft for pterygium". Cochrane Database Syst Rev. 2: CD011349. doi:10.1002/14651858.CD011349.pub2. PMID 26867004.
External links
 Media related to pterygium at Wikimedia Commons
Media related to pterygium at Wikimedia Commons- Facts About the Cornea and Corneal Disease The National Eye Institute (NEI)
- Pterygium eMedicine WebMD online article on pterygium (overview, differential diagnoses & workup, treatment & medication, and follow-up)
