T-HCA
 | |
| Clinical data | |
|---|---|
| Other names | trans-4-hydroxycrotonic acid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
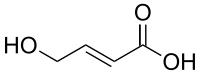
| Formula | C4H6O3 |
| Molar mass | 102.09 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
T-HCA, or trans-4-hydroxycrotonic acid, is a drug used in scientific research. It is structurally related to GHB and binds to the GHB receptor with 4-fold higher affinity than GHB itself,[1] but is not an agonist for the primary sedative target of GHB, the GABAB receptor, and so does not produce sedative effects, instead causing convulsions thought to be mediated through increased glutamate release.[2] It may also be naturally produced in the mammalian CNS and is suspected to be an endogenous ligand for the GHB receptor.[3]
See also
References
- ^ Wellendorph P, Høg S, Greenwood JR, de Lichtenberg A, Nielsen B, Frølund B, Brehm L, Clausen RP, Bräuner-Osborne H (2005). "Novel Cyclic γ-Hydroxybutyrate (GHB) Analogs with High Affinity and Stereoselectivity of Binding to GHB Sites in Rat Brain" (pdf). The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 346–351. doi:10.1124/jpet.105.090472. PMID 16014570.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Castelli MP, Ferraro L, Mocci I; et al. (2003). "Selective γ-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of γ-hydroxybutyric acid". Journal of Neurochemistry. 87 (3): 722–732. doi:10.1046/j.1471-4159.2003.02037.x. PMID 14535954.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Vayer P, Dessort D, Bourguignon JJ, Wermuth CG, Mandel P, Maitre M (1985). "Natural occurrence of trans-γ hydroxycrotonic acid in rat brain". Biochemical Pharmacology. 34 (13): 2401–2404. doi:10.1016/0006-2952(85)90804-4. PMID 4015683.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
