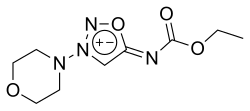
Molsidomine
Appearance

| |
| Names | |
|---|---|
| IUPAC name
1-Ethoxy-N-(3-morpholino-5-oxadiazol-3-iumyl)methanimidate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.042.902 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H14N4O4 | |
| Molar mass | 242.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Molsidomine is an orally active, long acting vasodilating drug. Molsidomine is metabolized in the liver to the active metabolite linsidomine. Linsidomine is an unstable compound that releases nitric oxide (NO) upon decay as the actual vasodilating compound.[1]
Release of nitric oxide
Molsidomine → Linsidomine (SIN-1) → SIN-1A → nitric oxide[2]
Chemistry
Molsidomine as well as linsidomine are sydnone imines, a class of mesoionic heterocyclic aromatic chemical compounds.
See also
References
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8743336, please use {{cite journal}} with
|pmid=8743336instead. - ^ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 558. ISBN 3-8047-1763-2.