Facioscapulohumeral muscular dystrophy: Difference between revisions
Lukelahood (talk | contribs) Populated infoboxes. Put in prognosis section. |
Lukelahood (talk | contribs) →Signs and symptoms: put in scoliosis |
||
| Line 61: | Line 61: | ||
Medical imaging (CT and MRI) has shown muscle damage that does not cause obvious symptoms.<ref name="Tasca2014MRIUpperGirdle">{{cite journal |last1=Tasca |first1=G |last2=Monforte |first2=M |last3=Iannaccone |first3=E |last4=Laschena |first4=F |last5=Ottaviani |first5=P |last6=Leoncini |first6=E |last7=Boccia |first7=S |last8=Galluzzi |first8=G |last9=Pelliccioni |first9=M |last10=Masciullo |first10=M |last11=Frusciante |first11=R |last12=Mercuri |first12=E |last13=Ricci |first13=E |title=Upper girdle imaging in facioscapulohumeral muscular dystrophy. |journal=PLOS ONE |date=2014 |volume=9 |issue=6 |pages=e100292 |doi=10.1371/journal.pone.0100292 |pmid=24932477|pmc=4059711 |bibcode=2014PLoSO...9j0292T }}</ref> A single MRI study shows the [[teres major muscle]] to be commonly affected.<ref name="Gerevini2016MRIWholeBody">{{cite journal |last1=Gerevini |first1=S |last2=Scarlato |first2=M |last3=Maggi |first3=L |last4=Cava |first4=M |last5=Caliendo |first5=G |last6=Pasanisi |first6=B |last7=Falini |first7=A |last8=Previtali |first8=SC |last9=Morandi |first9=L |s2cid=24650482 |title=Muscle MRI findings in facioscapulohumeral muscular dystrophy |journal=European Radiology |date=March 2016 |volume=26 |issue=3 |pages=693–705 |doi=10.1007/s00330-015-3890-1 |pmid=26115655}}</ref> The [[semimembranosus muscle]], part of the [[hamstrings]], is commonly affected,<ref name="Rijken2014CTWholeBody">{{cite journal |last1=Rijken |first1=NH |last2=van der Kooi |first2=EL |last3=Hendriks |first3=JC |last4=van Asseldonk |first4=RJ |last5=Padberg |first5=GW |last6=Geurts |first6=AC |last7=van Engelen |first7=BG |s2cid=101093 |title=Skeletal muscle imaging in facioscapulohumeral muscular dystrophy, pattern and asymmetry of individual muscle involvement. |journal=Neuromuscular Disorders |date=December 2014 |volume=24 |issue=12 |pages=1087–96 |doi=10.1016/j.nmd.2014.05.012 |pmid=25176503}}</ref><ref name="Mair2017MRILeg">{{cite journal |last1=Mair |first1=D |last2=Huegens-Penzel |first2=M |last3=Kress |first3=W |last4=Roth |first4=C |last5=Ferbert |first5=A |s2cid=25005883 |title=Leg Muscle Involvement in Facioscapulohumeral Muscular Dystrophy: Comparison between Facioscapulohumeral Muscular Dystrophy Types 1 and 2. |journal=European Neurology |date=2017 |volume=77 |issue=1–2 |pages=32–39 |doi=10.1159/000452763 |pmid=27855411}}</ref><ref name="Olsen2006MRILeg">{{cite journal |last1=Olsen |first1=DB |last2=Gideon |first2=P |last3=Jeppesen |first3=TD |last4=Vissing |first4=J |s2cid=19421344 |title=Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. |journal=Journal of Neurology |date=November 2006 |volume=253 |issue=11 |pages=1437–41 |doi=10.1007/s00415-006-0230-z |pmid=16773269}}</ref> deemed by one author to be "the most frequently and severely affected muscle."<ref name="Wagner2019Review" /> Also, MRI shows that of the quadricep muscles, the [[rectus femoris]] is preferentially affected;<ref name="Mair2017MRILeg" /> of the [[gastrocnemius]], the medial section is preferentially affected;<ref name="Mair2017MRILeg" /><ref name="Olsen2006MRILeg" /> and the [[iliopsoas]], a hip flexor muscle, is very often spared.<ref name="Olsen2006MRILeg" /><ref name="Wagner2019Review" /> |
Medical imaging (CT and MRI) has shown muscle damage that does not cause obvious symptoms.<ref name="Tasca2014MRIUpperGirdle">{{cite journal |last1=Tasca |first1=G |last2=Monforte |first2=M |last3=Iannaccone |first3=E |last4=Laschena |first4=F |last5=Ottaviani |first5=P |last6=Leoncini |first6=E |last7=Boccia |first7=S |last8=Galluzzi |first8=G |last9=Pelliccioni |first9=M |last10=Masciullo |first10=M |last11=Frusciante |first11=R |last12=Mercuri |first12=E |last13=Ricci |first13=E |title=Upper girdle imaging in facioscapulohumeral muscular dystrophy. |journal=PLOS ONE |date=2014 |volume=9 |issue=6 |pages=e100292 |doi=10.1371/journal.pone.0100292 |pmid=24932477|pmc=4059711 |bibcode=2014PLoSO...9j0292T }}</ref> A single MRI study shows the [[teres major muscle]] to be commonly affected.<ref name="Gerevini2016MRIWholeBody">{{cite journal |last1=Gerevini |first1=S |last2=Scarlato |first2=M |last3=Maggi |first3=L |last4=Cava |first4=M |last5=Caliendo |first5=G |last6=Pasanisi |first6=B |last7=Falini |first7=A |last8=Previtali |first8=SC |last9=Morandi |first9=L |s2cid=24650482 |title=Muscle MRI findings in facioscapulohumeral muscular dystrophy |journal=European Radiology |date=March 2016 |volume=26 |issue=3 |pages=693–705 |doi=10.1007/s00330-015-3890-1 |pmid=26115655}}</ref> The [[semimembranosus muscle]], part of the [[hamstrings]], is commonly affected,<ref name="Rijken2014CTWholeBody">{{cite journal |last1=Rijken |first1=NH |last2=van der Kooi |first2=EL |last3=Hendriks |first3=JC |last4=van Asseldonk |first4=RJ |last5=Padberg |first5=GW |last6=Geurts |first6=AC |last7=van Engelen |first7=BG |s2cid=101093 |title=Skeletal muscle imaging in facioscapulohumeral muscular dystrophy, pattern and asymmetry of individual muscle involvement. |journal=Neuromuscular Disorders |date=December 2014 |volume=24 |issue=12 |pages=1087–96 |doi=10.1016/j.nmd.2014.05.012 |pmid=25176503}}</ref><ref name="Mair2017MRILeg">{{cite journal |last1=Mair |first1=D |last2=Huegens-Penzel |first2=M |last3=Kress |first3=W |last4=Roth |first4=C |last5=Ferbert |first5=A |s2cid=25005883 |title=Leg Muscle Involvement in Facioscapulohumeral Muscular Dystrophy: Comparison between Facioscapulohumeral Muscular Dystrophy Types 1 and 2. |journal=European Neurology |date=2017 |volume=77 |issue=1–2 |pages=32–39 |doi=10.1159/000452763 |pmid=27855411}}</ref><ref name="Olsen2006MRILeg">{{cite journal |last1=Olsen |first1=DB |last2=Gideon |first2=P |last3=Jeppesen |first3=TD |last4=Vissing |first4=J |s2cid=19421344 |title=Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. |journal=Journal of Neurology |date=November 2006 |volume=253 |issue=11 |pages=1437–41 |doi=10.1007/s00415-006-0230-z |pmid=16773269}}</ref> deemed by one author to be "the most frequently and severely affected muscle."<ref name="Wagner2019Review" /> Also, MRI shows that of the quadricep muscles, the [[rectus femoris]] is preferentially affected;<ref name="Mair2017MRILeg" /> of the [[gastrocnemius]], the medial section is preferentially affected;<ref name="Mair2017MRILeg" /><ref name="Olsen2006MRILeg" /> and the [[iliopsoas]], a hip flexor muscle, is very often spared.<ref name="Olsen2006MRILeg" /><ref name="Wagner2019Review" /> |
||
===Non- |
===Non-muscular=== |
||
The most common non-musculoskeletal manifestation of FSHD is mild retinal blood vessel abnormalities, such as telangiectasias or microaneurysms, with one study placing the incidence at 50%.<ref name="Padbert1995Retinopathy">{{cite journal |last1=Padberg |first1=G. W. |last2=Brouwer |first2=O. F. |last3=de Keizer |first3=R. J. W. |last4=Dijkman |first4=G. |last5=Wijmenga |first5=C. |last6=Grote |first6=J. J. |last7=Frants |first7=R. R. |title=On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy |journal=Muscle & Nerve |date=1995 |volume=18 |issue=S13 |pages=S73–S80 |doi=10.1002/mus.880181314|hdl=2066/20764 |hdl-access=free }}</ref> These abnormal blood vessels generally do not affect vision or health, although a severe form of it mimics [[Coat's disease]], a condition found in about 1% of FSHD cases and more frequently associated with large 4q35 deletions.<ref name="Wagner2019Review" /><ref>{{Cite journal|last1=Lindner|first1=Moritz|last2=Holz|first2=Frank G|last3=Charbel Issa|first3=Peter|date=2016-04-27|title=Spontaneous resolution of retinal vascular abnormalities and macular oedema in facioscapulohumeral muscular dystrophy|journal=Clinical & Experimental Ophthalmology|language=en|volume=44|issue=7|pages=627–628|doi=10.1111/ceo.12735|pmid=26933772|s2cid=204996841|issn=1442-6404}}</ref> High-frequency hearing loss can occur in those with large 4q35 deletions, but otherwise is no more common compared to the general population.<ref name="Wagner2019Review" /> Breathing can be affected, associated with [[kyphoscoliosis]] and wheelchair use; it is seen in one-third of wheelchair-bound patients.<ref name="Wagner2019Review" /> However, ventilator support (nocturnal or diurnal) is needed in only 1% of cases.<ref name="Wagner2019Review" /><ref>{{cite journal |vauthors=Wohlgemuth M, van der Kooi EL, van Kesteren RG, van der Maarel SM, Padberg GW | s2cid = 31335126 | year = 2004 | title = Ventilatory support in facioscapulohumeral muscular dystrophy | journal = Neurology | volume = 63 | issue = 1| pages = 176–8 | pmid = 15249635 | doi=10.1212/01.wnl.0000133126.86377.e8| citeseerx = 10.1.1.543.2968 }}</ref> Although there are reports of increased risk of cardiac arrhythmias, general consensus is that the heart is not affected.<ref name="Tawil2006Review" /> |
The most common non-musculoskeletal manifestation of FSHD is mild retinal blood vessel abnormalities, such as telangiectasias or microaneurysms, with one study placing the incidence at 50%.<ref name="Padbert1995Retinopathy">{{cite journal |last1=Padberg |first1=G. W. |last2=Brouwer |first2=O. F. |last3=de Keizer |first3=R. J. W. |last4=Dijkman |first4=G. |last5=Wijmenga |first5=C. |last6=Grote |first6=J. J. |last7=Frants |first7=R. R. |title=On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy |journal=Muscle & Nerve |date=1995 |volume=18 |issue=S13 |pages=S73–S80 |doi=10.1002/mus.880181314|hdl=2066/20764 |hdl-access=free }}</ref> These abnormal blood vessels generally do not affect vision or health, although a severe form of it mimics [[Coat's disease]], a condition found in about 1% of FSHD cases and more frequently associated with large 4q35 deletions.<ref name="Wagner2019Review" /><ref>{{Cite journal|last1=Lindner|first1=Moritz|last2=Holz|first2=Frank G|last3=Charbel Issa|first3=Peter|date=2016-04-27|title=Spontaneous resolution of retinal vascular abnormalities and macular oedema in facioscapulohumeral muscular dystrophy|journal=Clinical & Experimental Ophthalmology|language=en|volume=44|issue=7|pages=627–628|doi=10.1111/ceo.12735|pmid=26933772|s2cid=204996841|issn=1442-6404}}</ref> High-frequency hearing loss can occur in those with large 4q35 deletions, but otherwise is no more common compared to the general population.<ref name="Wagner2019Review" /> [[Scoliosis]] can occur, though to result from weakness of abdominal, hip extensor, and spinal muscles.<ref name="Eren2020SpinalFusion">{{cite journal |last1=Eren |first1=İ |last2=Abay |first2=B |last3=Günerbüyük |first3=C |last4=Çakmak |first4=ÖÖ |last5=Şar |first5=C |last6=Demirhan |first6=M |title=Spinal fusion in facioscapulohumeral dystrophy for hyperlordosis: A case report. |journal=Medicine |date=February 2020 |volume=99 |issue=8 |pages=e18787 |doi=10.1097/MD.0000000000018787 |pmid=32080072}}</ref><ref name="Huml2015Review">{{cite journal |last1=Huml |first1=Raymond A. |last2=Perez |first2=Daniel P. |title=FSHD: The Most Common Type of Muscular Dystrophy? |journal=Muscular Dystrophy |date=2015 |pages=9–19 |doi=doi.org/10.1007/978-3-319-17362-7_3}}</ref> Conversely, scoliosis can be viewed as a compensatory mechanism to weakness.<ref name="Eren2020SpinalFusion" /> Breathing can be affected, associated with [[kyphoscoliosis]] and wheelchair use; it is seen in one-third of wheelchair-bound patients.<ref name="Wagner2019Review" /> However, ventilator support (nocturnal or diurnal) is needed in only 1% of cases.<ref name="Wagner2019Review" /><ref>{{cite journal |vauthors=Wohlgemuth M, van der Kooi EL, van Kesteren RG, van der Maarel SM, Padberg GW | s2cid = 31335126 | year = 2004 | title = Ventilatory support in facioscapulohumeral muscular dystrophy | journal = Neurology | volume = 63 | issue = 1| pages = 176–8 | pmid = 15249635 | doi=10.1212/01.wnl.0000133126.86377.e8| citeseerx = 10.1.1.543.2968 }}</ref> Although there are reports of increased risk of cardiac arrhythmias, general consensus is that the heart is not affected.<ref name="Tawil2006Review" /> |
||
==Genetics== |
==Genetics== |
||
Revision as of 00:34, 29 May 2021
| Facioscapulohumeral muscular dystrophy | |
|---|---|
| Other names | Landouzy–Dejerine muscular dystrophy, FSHMD, FSH |
 | |
| A diagram showing in red the muscles noticeably involved in FSHD | |
| Pronunciation | |
| Specialty | Neurology, neuromuscular medicine |
| Symptoms | Facial weakness, scapular winging, foot drop |
| Complications | Chronic pain, scoliosis Rare: respiratory insufficiency, hearing loss, retinal disease |
| Usual onset | Ages 15 – 30 years |
| Duration | Lifelong |
| Types | FSHD1, FSHD2, infantile-onset |
| Causes | Genetic (inherited or new mutation) |
| Risk factors | Male sex |
| Diagnostic method | Genetic testing |
| Differential diagnosis | Limb-girdle muscular dystrophy (especially calpainopathy), Pompe disease, mitochondrial myopathy, polymyositis[2] |
| Management | Physical therapy, bracing, reconstructive surgery |
| Medication | Clinical trials ongoing |
| Prognosis | Progressive, unaffected life expectancy |
| Frequency | Up to 8,333[2] |
Facioscapulohumeral muscular dystrophy (FSHD) is a type of muscular dystrophy, a group of heritable diseases that cause progressive and irreversible impairment of muscles. FSHD preferentially weakens the skeletal muscles of the face (Latin: facio), those that position the scapula (scapulo), and those in the upper arm, overlying the humerus bone (humeral).[2] These areas can be spared, and others usually are affected, especially the chest, the trunk, and around the shin. Abnormally positioned scapulas (winged scapulas) and inability to lift the foot (foot drop) are common. The two sides of the body are often affected unequally. Weakness typically manifests at ages 15 – 30 years.[3] FSHD can also cause hearing loss and blood vessel abnormalities in the back of the eye.
FSHD is caused by complex genetic changes involving the DUX4 gene.[4] Normally, DUX4 is expressed (i.e., turned on) in cells of the ovary and in very early human development, becoming repressed (i.e., turned off) by the time an embryo is several days old.[5][6] In FSHD, a genetic mutation causes DUX4 to be inadequately repressed, allowing expression throughout life. Deletion of DNA in the region surrounding DUX4 is the causative mutation in 95% of cases, termed "D4Z4 contraction" and defining FSHD type 1 (FSHD1).[7] FSHD caused by other mutations is FSHD type 2 (FSHD2). For disease to develop, also required is a 4qA allele, which is a common variation in the DNA next to DUX4. The chances of a D4Z4 contraction with a 4qA allele being passed on to children is 50% (autosomal dominant);[2] in 30% of cases, the mutation arises spontaneously.[3] Mutations of FSHD cause decreased DNA methylation, making DUX4 accessible to be copied into messenger RNA (mRNA). The 4qA allele stabilizes DUX4 mRNA, allowing it to be used for production of DUX4 protein.[8] DUX4 protein is a modulator of hundreds of other genes, many of which are involved in muscle function.[2][4] How this genetic modulation causes muscle damage remains unclear.[2] Diagnosis is by genetic testing.[2]
No intervention has proven effective for slowing progression of weakness. Screening can be done for various disease complications. Symptoms can be addressed with physical therapy, bracing, and reconstructive surgery such as surgical fixation of the scapula to the thorax.[9] FSHD affects up to 1 in 8,333 people.[2] It is the third most common muscular dystrophy, Duchenne muscular dystrophy being first and myotonic dystrophy being second. Prognosis is variable. Many are not significantly limited in daily activity. A wheel chair or scooter is required in 20% of cases.[10] Life expectancy is generally not affected, except in rare cases of respiratory insufficiency.[11]
FSHD was first distinguished as a disease in the 1870s and 1880s when French physicians Landouzy and Dejerine followed a family affected by it, thus the initial name Landouzy–Dejerine muscular dystrophy. However, their work is predated by descriptions of probable individual FSHD cases.[12][13][14] The significance of D4Z4 contraction on chromosome 4 was established in the 1990s. The DUX4 gene was discovered in 1999, found to be expressed and toxic in 2007, and in 2010 the genetic mechanism causing its expression was elucidated. In 2012, the predominant mutation of FSHD2 was discovered. In 2019, the first drug designed to counteract DUX4 expression entered clinical trials.
Signs and symptoms
Muscles of the face, shoulder girdle, and upper arm are classically affected, although these muscles can be spared and other muscles usually are affected. Distribution and degree of muscle weakness is extremely variable, even between identical twins.[15][16] Individual muscles can weaken while adjacent muscles remain healthy.[citation needed] Muscle weakness usually becomes noticeable on one side of the body before the other, a hallmark of the disease.[10] The right shoulder and arm muscles are more often affected than the left upper extremity muscles, independent of handedness.[17]: 139 [18] Otherwise, muscle involvement is equally common on both sides.[citation needed] Musculoskeletal pain is very common, most often described in the neck, shoulders, lower back, and the back of the knee.[19] Classically, symptoms appear in those 15 – 30 years of age, although infantile onset, adult onset, and absence of symptoms despite having the causal genetics also occur.[3] Disease progression is slow, and long static phases, in which no progression is apparent, is not uncommon.[20] Less commonly, rapid deterioration of individual muscles over several months can occur.[2] FSHD1 and FSHD2 are indistinguishable on basis of signs and symptoms,[21] although very large D4Z4 deletions in FSHD1 (EcoRI 10-11 kb) are more strongly associated with infantile onset, progressive hearing loss, retinal disease, and various rare manifestations.[22]
Face and shoulder
Weakness of the muscles of the face is the most distinguishing sign of FSHD.[3] It is typically earliest sign, although it is rarely the initial complaint.[3] At least mild facial weakness can be found in 90% or more with FSHD.[20][23] The most commonly affected facial muscles are those responsible for closing the eyelids, pursing the lips, and raising the corners of the mouth (smile), which respectively are the orbicularis oculi, orbicularis oris, and zygomaticus major muscles.[3] Orbicularis oculi weakness can result in sleeping with eyelids open and dry eyes.[3] Orbicularis oris weakness can result in inability to pucker the lips or whistle.[3] Zygomaticus major weakness predisposes one to a "horizontal smile," which looks more like a grin.[3] Weakness of various facial muscles contributes to difficulty pronouncing the letters M, B, and P.[citation needed] Facial expressions can appear diminished, arrogant, grumpy, or fatigued.[3] Muscles used for chewing and moving the eyes are not affected.[10] FSHD is generally progressive, but it is not established whether facial weakness is progressive or stable throughout life.[24]
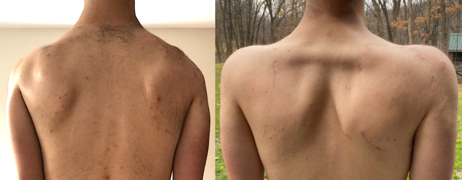
After the facial weakness, weakness usually develops in the muscles of the chest and those that connect the scapula to the thorax. Symptoms involving the shoulder, such as difficulty working with the arms overhead, are the initial complaint in 80% of cases.[23][10] Predominantly, the serratus anterior and middle and lower trapezii muscles are affected;[3] the upper trapezius is often spared.[10] Trapezius weakness causes the scapulas to become downwardly rotated and protracted, resulting in winged scapulas, horizontal clavicles, and sloping shoulders. Severe muscle wasting can make bones and spared shoulder muscles very visible, a characteristic example being the "poly-hill" sign.[3] The first "hill" or bump is the upper corner of scapula appearing to "herniate" up and over the rib cage. The second hill is the AC joint, seen between a wasted upper trapezius and wasted upper deltoid. The third hill is the lower deltoid, distinguishable between the wasted upper deltoid and wasted humeral muscles.[3] Also affected is the chest, particularly the parts of the pectoralis major muscle that connect to the sternum and ribs. The part that connects to the clavicle is less often affected. This muscle wasting pattern can contribute to a prominent horizontal anterior axillary fold.[25][3] Muscles connecting the arm to the scapula are generally spared, which include deltoid and the rotator cuff muscles.[26][27] The deltoid can be affected later on, especially the upper portion.[3] Beyond this point the disease does not progress further in 30% of familial cases.[23][10]
Upper arm and lower body
After facial and upper torso weakness, weakness can "descend" to the upper arms (biceps muscle and triceps muscle) and the pelvic girdle.[20] The forearms are usually spared, resulting in an appearance some compare to the fictional character Popeye.[3] Sometimes, the weakness is observed to "skip" the pelvis and involve the tibialis anterior (shin muscle), causing foot drop. Weakness can also occur in the abdominal muscles, which can manifest as a protuberant abdomen, lumbar hyperlordosis, the inability to do a sit-up, or the inability to turn from one side to the other while lying down. Of the rectus abdominis muscle, the lower portion is preferentially affected, manifesting as a positive Beevor's sign.[3] Weakness in the legs can manifest as difficulty walking or hips held in slight flexion.
Muscle involvement from medical imaging perspective
Medical imaging (CT and MRI) has shown muscle damage that does not cause obvious symptoms.[26] A single MRI study shows the teres major muscle to be commonly affected.[27] The semimembranosus muscle, part of the hamstrings, is commonly affected,[18][28][29] deemed by one author to be "the most frequently and severely affected muscle."[2] Also, MRI shows that of the quadricep muscles, the rectus femoris is preferentially affected;[28] of the gastrocnemius, the medial section is preferentially affected;[28][29] and the iliopsoas, a hip flexor muscle, is very often spared.[29][2]
Non-muscular
The most common non-musculoskeletal manifestation of FSHD is mild retinal blood vessel abnormalities, such as telangiectasias or microaneurysms, with one study placing the incidence at 50%.[30] These abnormal blood vessels generally do not affect vision or health, although a severe form of it mimics Coat's disease, a condition found in about 1% of FSHD cases and more frequently associated with large 4q35 deletions.[2][31] High-frequency hearing loss can occur in those with large 4q35 deletions, but otherwise is no more common compared to the general population.[2] Scoliosis can occur, though to result from weakness of abdominal, hip extensor, and spinal muscles.[32][33] Conversely, scoliosis can be viewed as a compensatory mechanism to weakness.[32] Breathing can be affected, associated with kyphoscoliosis and wheelchair use; it is seen in one-third of wheelchair-bound patients.[2] However, ventilator support (nocturnal or diurnal) is needed in only 1% of cases.[2][34] Although there are reports of increased risk of cardiac arrhythmias, general consensus is that the heart is not affected.[10]
Genetics

The genetics of FSHD is unique and complex, centering around the DUX4 gene. Normally, DUX4 is expressed during embryogenesis and later repressed in all tissues except the testes. In FSHD, there is failure of DUX4 repression and continued production of DUX4 protein, which is toxic to muscles.[2][7] The mechanism of failed DUX4 repression is hypomethylation of DUX4 and its surrounding DNA on the tip of chromosome 4 (4q35), allowing transcription of DUX4 into messenger RNA (mRNA). Several mutations can result in disease, upon which FSHD is sub-classified into FSHD type 1 (FSHD1) and FSHD type 2 (FSHD2).[21] Disease can only result when a mutation is present in combination with select, commonly found variations of 4q35, termed haplotype polymorphisms. There are at least 17 4q35 haplotype polymorphisms,[35] roughly dividable into the groups 4qA and 4qB.[35] A 4qA haplotype polymorphism, often referred to as a 4qA allele, is necessary for disease, as it contains a polyadenylation sequence that allows DUX4 mRNA to resist degradation long enough to be translated into DUX4 protein.[7]
DUX4 and the D4Z4 repeat array

| CEN | centromeric end | TEL | telomeric end |
| NDE box | non-deleted element | PAS | polyadenylation site |
| triangle | D4Z4 repeat | trapezoid | partial D4Z4 repeat |
| white box | pLAM | gray boxes | DUX4 exons 1, 2, 3 |
| arrows | |||
| corner | promoters | straight | RNA transcripts |
| black | sense | red | antisense |
| blue | DBE-T | dashes | dicing sites |
DUX4 resides within the D4Z4 macrosatellite repeat array, a series of tandemly repeated DNA segments in the subtelomeric region (4q35) of chromosome 4. Each D4Z4 repeat is 3.3 kilobase pairs (kb) long and is the site of epigenetic regulation, containing both heterochromatin and euchromatin structures.[36][37] In FSHD, the heterochromatin structure is lost, becoming euchromatin.[36] The name "D4Z4" is derived from an obsolete nomenclature system used for DNA segments of unknown significance during the human genome project: D for DNA, 4 for chromosome 4, Z indicates it is a repetitive sequence, and 4 is a serial number assigned based on the order of submission.[38][39]
The subtelomeric region of chromosome 10q contains a tandem repeat structure highly homologous (99% identical) to 4q35,[7][35] containing "D4Z4-like" repeats with protein-coding regions identical to DUX4 (D10Z10 repeats and DUX4L10, respectively).[7][40] Because 10q usually lacks a polyadenylation sequence, it is usually not implicated in disease. However, chromosomal rearrangements can occur between 4q and 10q repeat arrays, and involvement in disease is possible if a 4q D4Z4 repeat and polyadenylation signal are transferred onto 10q,[41][7][42] or if rearrangement causes FSHD1.
DUX4 consists of three exons. Exons 1 and 2 are in each repeat. Exon 3 is in the pLAM region telomeric to the last partial repeat.[7][6] Multiple RNA transcripts are produced from the D4Z4 repeat array, both sense and antisense. Some transcripts might be degraded in areas to produce si-like small RNAs.[21] Some transcripts that originate centromeric to the D4Z4 repeat array at the non-deleted element (NDE), termed D4Z4 regulatory element transcripts (DBE-T), could play a role in DUX4 derepression.[21][43] One proposed mechanism is that DBE-T leads to the recruitment of the trithorax-group protein Ash1L, an increase in H3K36me2-methylation, and ultimately de-repression of 4q35 genes.[44]
FSHD1
FSHD involving deletion of D4Z4 repeats (termed 'D4Z4 contraction') on 4q is classified as FSHD1, which accounts for 95% of FSHD cases.[2] Typically, chromosome 4 includes between 11 and 150 D4Z4 repeats.[36][7] In FSHD1, there are 1–10 D4Z4 repeats.[7] The number of repeats is roughly inversely related to disease severity. Namely, those with 8 - 10 repeats tend to have the mildest presentations, sometimes with no symptoms; those with 4 - 7 repeats have moderate disease that is highly variable; and those with 1 - 3 repeats are more likely to have severe, atypical, and early-onset disease.[45] Deletion of the entire D4Z4 repeat array does not result in FSHD because then there are no complete copies of DUX4 to be expressed, although other birth defects result.[46][7] One contracted D4Z4 repeat array with an adjoining 4qA allele is sufficient to cause disease, so inheritance is autosomal dominant. De novo (new) mutations are implicated in 10 - 30% of cases,[3] 50% of which exhibit somatic mosaicism.[citation needed]
It has been proposed that FSHD1 undergoes anticipation, a trend primarily associated with trinucleotide repeat disorders in which disease manifestation worsens with each subsequent generation.[47] As of 2019, more detailed studies are needed to definitively show whether or not anticipation plays a role.[48] If anticipation does occur in FSHD, the mechanism is different than that of trinucleotide repeat disorders, since D4Z4 repeats are much larger than trinucleotide repeats, an underabundance of repeats (rather than overabundance) causes disease, and the repeat array size in FSHD is stable across generations.[49]
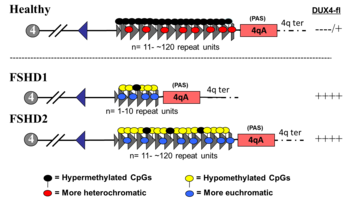
FSHD2
FSHD without D4Z4 contraction is classified as FSHD2, which constitutes 5% of FSHD cases.[2] Various mutations cause FSHD2, all resulting in D4Z4 hypomethylation, at which the genetic mechanism converges with FSHD1.[50] Approximately 80% of FSHD2 cases are due to deactivating mutations in the gene SMCHD1 (structural maintenance of chromosomes flexible hinge domain containing 1) on chromosome 18. SMCHD1 is responsible for DNA methylation, and its deactivation results in hypomethylation of the D4Z4 repeat array.[2] Another cause of FSHD2 is mutation in DNMT3B (DNA methyltransferase 3B), which also plays a role in DNA methylation.[51][52] As of 2020, early evidence indicates that a third cause of FSHD2 is mutation in both copies of the LRIF1 gene, which encodes the protein ligand-dependent nuclear receptor-interacting factor 1 (LRIF1).[53] LRIF1 is known to interact with the SMCHD1 protein.[53] As of 2019, there are presumably additional mutations at other unidentified genetic locations that can cause FSHD2.[2]
Mutation of a single allele of SMCHD1 or DNMT3B can cause disease. Mutation of both copies LRIF1 has been tentatively shown to cause disease in a single person as of 2020.[53] As in FSHD1, a 4qA allele must be present for disease to result. However, unlike the D4Z4 array, the genes implicated in FSHD2 are not in proximity with the 4qA allele, and so they are inherited independently from the 4qA allele, resulting in a digenic inheritance pattern. For example, one parent without FSHD can pass on an SMCHD1 mutation, and the other parent, also without FSHD, can pass on a 4qA allele, bearing a child with FSHD2.[50][52]
Two ends of a disease spectrum
Initially, FSHD1 and FSHD2 were described as two separate genetic causes of the same disease. However, they can also be viewed not as distinct causes, but rather as risk factors. Not rarely do both contribute to disease in the same individual.[45]
In those with FSHD2, although they have do not have a 4qA allele with D4Z4 repeat number less than 11, they still often have one less than 17 (relatively short compared to the general population), suggesting that a large number of D4Z4 repeats can prevent the effects of an SMCHD1 mutation.[45] Further studies need to be done to determine the upper limit of D4Z4 repeats in which FSHD2 can occur.[45]
In those with a 4qA allele and 10 or fewer repeats, an additional SMCHD1 mutation has shown to worsen disease, classifying them as both FSHD1 and FSHD2.[54] In these FSHD1/FSHD2 individuals, the methylation pattern of the D4Z4 repeat array resembles that seen in FSHD2.[45] This combined FSHD1/FSHD2 presentation is most common in those with 9 - 10 repeats, and is seldom found in those with 8 or less repeats. The relative abundance of SMCHD1 mutations in the 9 - 10 repeat group is likely because a sizable portion of the general population has 9 - 10 repeats with no disease, yet with the additive effect of an SMCHD1 mutation, symptoms develop and a diagnosis is made. In those with 8 or fewer repeats, symptoms are more likely than in those with 9 - 10 repeats, leading to diagnosis regardless of an additional SMCHD1 mutation.[45]
The apparent frequency of FSHD1/FSHD2 cases in the 9 - 10 repeat range, combined with the FSHD2-like methylation pattern, suggest the 9 - 10 repeat size to be an overlap zone between FSHD1 and FSDH2.[45]
Pathophysiology
Molecular

As of 2020, there seems to be a consensus that aberrant expression of DUX4 in muscle is the cause of FSHD.[55] DUX4 is expressed in extremely small amounts, detectable in 1 out of every 1000 immature muscle cells (myoblast), which appears to increase after myoblast maturation, in part because the cells fuse as they mature, and a single nucleus expressing DUX4 can provide DUX4 protein to neighboring nuclei from fused cells.[56]
It remains an area of active research how DUX4 protein causes muscle damage. DUX4 protein is a transcription factor that regulates many other genes. Some of these genes are involved in apoptosis, such as p53, p21, MYC, and β-catenin. It seems that DUX4 protein makes muscle cells more prone to apoptosis, although details of the mechanism are still unknown and contested. Other DUX4 protein-regulated genes are involved in oxidative stress, and it seems that DUX4 expression lowers muscle cell tolerance of oxidative stress. Variation in the ability of individual muscles to handle oxidative stress could partially explain the muscle involvement patterns of FSHD. DUX4 protein downregulates many genes involved in muscle development, including MyoD, myogenin, desmin, and PAX7. DUX4 expression has shown to reduce muscle cell proliferation, differentiation, and fusion. Estrogen seems to play a role on in modifying DUX4 protein effects on muscle differentiation, which could explain why females are less affected than males. DUX4 protein regulates a few genes that are involved in RNA quality control, and DUX4 expression has been shown to cause accumulation of RNA with subsequent apoptosis.[55]
The cellular hypoxia response has been reported in a single study to be the main driver of DUX4 protein-induced muscle cell death. The hypoxia-inducible factors (HIFs) are upregulated by DUX4 protein, possibly causing pathologic signaling leading to cell death.[57]
Another study found that DUX4 expression in muscle cells led to the recruitment and alteration of fibrous/fat progenitor cells, which helps explain why muscles become replaced by fat and fibrous tissue.[56]
Muscle
Unlike other muscular dystrophies, early muscle biopsies show only mild degrees of fibrosis, muscle fiber hypertrophy, and displacement of nuclei from myofiber peripheries (central nucleation).[21] More often found is inflammation.[21] There can be endomysial inflammation, primarily composed of CD8+ T-cells, although these cells do not seem to directly cause muscle fiber death.[21] Endomysial blood vessels can be surrounded by inflammation, which is relatively unique to FSHD, and this inflammation contains CD4+ T-cells.[21] Inflammation is succeeded by deposition of fat (fatty infiltration), then fibrosis.[58][21]
Why certain muscles are preferentially affected in FSHD remains unknown. One interesting observation is that the right shoulder and arm muscles are more often affected than the left upper extremity muscles, a pattern also seen in Poland syndrome and hereditary neuralgic amyotrophy; this could reflect a genetic, developmental/anatomic, or functional-related mechanism.[23][18]
Another unexplained muscle involvement pattern raised by one researcher is the sparing of the deltoid, which is not seen in any other condition that affects the muscles around the scapula.[24]
Diagnosis
Genetic testing
Genetic testing is the gold standard for FSHD diagnosis, as it is the most sensitive and specific test available.[2] Commonly, FSHD1 is tested for first.[2] A shortened D4Z4 array length (EcoRI length of 10 kb to 38 kb) with an adjacent 4qA allele supports FSHD1.[2] If FSHD1 is not present, commonly FSHD2 is tested for next by assessing methylation at 4q35.[2] Low methylation (less than 20%) in the context of a 4qA allele is sufficient for diagnosis.[2] The specific mutation, usually one of various SMCHD1 mutations, can be identified with next-generation sequencing (NGS).[59]
Assessing D4Z4 length
Measuring D4Z4 length is technically challenging due to the D4Z4 repeat array consisting of long, repetitive elements.[60] For example, NGS is not useful for assessing D4Z4 length, because it breaks DNA into fragments before reading them, and it is unclear from which D4Z4 repeat each sequenced fragment came.[3] In 2020, optical mapping became available for measuring D4Z4 array length, which is more precise and less labor intensive than southern blot.[61] Molecular combing is also available for assessing D4Z4 array length.[62] Sometimes 4q or 10q will have a combination of D4Z4 and D4Z4-like repeats due to DNA exchange between 4q and 10q, which can yield erroneous results, requiring more detailed workup.[35]

Restriction fragment length polymorphism (RFLP) analysis was the first genetic test developed and is still used as of 2020, although it is being phased out by newer methods. It involves dicing the DNA with restriction enzymes and sorting the resulting restriction fragments by size using southern blot. The restriction enzymes EcoRI and BlnI are commonly used. EcoRI isolates the 4q and 10q repeat arrays, and BlnI dices the 10q sequence into small pieces, allowing 4q to be distinguished.[3][35] The EcoRI restriction fragment is composed of three parts: 1) 5.7 kb proximal part, 2) the central, variable size D4Z4 repeat array, and 3) the distal part, usually 1.25 kb.[63] The proximal portion has a sequence of DNA stainable by the probe p13E-11, which is commonly used to visualize the EcoRI fragment during southern blot.[35] The name "p13E-11" reflects that it is a subclone of a DNA sequence designated as cosmid 13E during the human genome project.[64][65] Sometimes D4Z4 repeat array deletions can include the p13E-11 binding site, warranting use of alternate probes.[35] Considering that each D4Z4 repeat is 3.3 kb, and the EcoRI fragment contains 6.9 kb of DNA that is not part of the D4Z4 repeat array, the number of D4Z4 units can be calculated.
- D4Z4 repeats = (EcoRI length - 6.9) / 3.3
Alternative testing

Other tests can support the diagnosis of FSHD, although they are all less sensitive and less specific than genetic testing.[66]
- Creatine kinase (CK) blood level is often ordered when muscle damage is suspected. CK is an enzyme found in muscle, leaking into the blood when muscles become damaged. In FSHD, CK level is normal to mildly elevated.[2]
- Electromyogram (EMG) measures the electrical activity in the muscle. EMG can show nonspecific signs of muscle damage or irritability.[2]
- Nerve conduction velocity (NCV) measures the how fast signals travel along a nerve. The nerve signals are measured with surface electrodes (similar to those used for an electrocardiogram) or needle electrodes.
- Muscle biopsy is the surgical removal and examination of a small piece of muscle, usually from the arm or leg. Microscopy and a variety of biochemical tests are used for examination. Findings in FSHD are nonspecific, such as presence of white blood cells or variation in muscle fiber size. This test is rarely indicated.[2]
- Muscle MRI is sensitive for detecting muscle damage, even in mild cases. Because of the particular muscle involvement patterns of FSHD, MRI can help differentiate FSHD from other muscle diseases, directing genetic testing.[26][27]
Management
No intervention has definitively proven to significantly alter the disease progression.
Screening and monitoring of complications
Multiple medical tests can be done to detect complications. A dilated eye exam to look for retinal abnormalities is recommended in those newly diagnosed with FSHD. Those with large D4Z4 deletions should be referred to a retinal specialist for yearly exams.[67][2] A hearing test should be done in individuals with early-onset FSHD, prior to starting school, or any other FSHD-affected individual with symptoms of hearing loss.[67][2] Pulmonary function testing (PFT) should be done in those newly diagnosed to establish baseline pulmonary function.[2] PFT should also be done recurrently for those with risks for or symptoms of pulmonary insufficiency.[67][2]
Physical and occupational therapy
Aerobic exercise has been shown to reduce chronic fatigue and decelerate fatty infiltration of muscle in FSHD.[68][69] The American Academy of Neurology (ANN) recommends that people with FSHD engage in low-intensity aerobic exercise to promote energy levels, muscle health, and bone health.[2] Moderate-intensity strength training appears to do no harm, although it has not been shown to be beneficial.[70] Physical therapy can address specific symptoms; there is no standardized protocol for FSHD. Anecdotal reports suggest that appropriately applied kinesiology tape can reduce pain.[71] Occupational therapy can be used for training in activities of daily living (ADLs) and to help adapt to new assistive devices. Cognitive behavioral therapy (CBT) has been shown to reduce chronic fatigue in FSHD, and it also decelerates fatty infiltration of muscle when directed towards increasing daily activity.[68][69]
Braces are often used to address muscle weakness. Scapular bracing can improve scapular positioning, which improves shoulder function, although it is often deemed as ineffective or impractical.[72] Ankle-foot orthoses can improve walking, balance, and quality of life.[73]
Pharmacologic management
As of 2021, no pharmaceuticals have definitively proven effective for altering the disease course. Although some pharmaceuticals have shown improved muscle mass, they did not improve quality of life.
Reconstructive surgery
Various manifestations of facial weakness are amenable to surgical correction. Upper eyelid gold implants have been used for those unable to close their eyes.[74] Drooping lower lip has been addressed with plastic surgery.[75] Select cases of foot drop can be surgically corrected with tendon transfer. The tibialis posterior muscle is repurposed as a tibialis anterior muscle, a version of this being called the Bridle procedure.[76][77][71] Severe scoliosis caused by FSHD can be corrected with spinal fusion; however, since scoliosis is a compensatory change in response to muscle weakness, correction of spinal alignment can result in further impaired muscle function.
Scapular winging in amenable to several procedures, the best known being scapulothoracic fusion (arthrodesis), an orthopedic procedure that achieves bony fusion between the scapula and the ribs. It increases shoulder active range of motion, improves shoulder function, decreases pain, and improves cosmetic appearance.[78][79] Active range of motion increases most in the setting of severe scapular winging with an unaffected deltoid muscle;[9] however, passive range of motion decreases. Namely, the patient gains the ability to slowly flex and abduct their shoulders to 90+ degrees, but they lose the ability to "throw" their arm up to a full 180 degrees.[2] A second procedure type is scapulopexy, which involves tethering the scapula to the ribs, vertebrae, or other scapula using tendon grafts, wire, or other means. Unlike the scapulothoracic fusion, no fusion between bones is achieved. Scapulopexy is considered to be less invasive than scapulothoracic fusion, but more susceptible to long-term failure. An alternative treatment, not commonly done, is tendon transfer, which involves rearranging the attachments of muscles to bone. Examples include pectoralis major transfer and the Eden-Lange procedure.[80]
- Scapular winging management
-
Kinesiology tape applied across the scapulas.
-
A cloth brace to hold the scapulas in retraction to reduce shoulder symptoms, such as collarbone pain.
-
Scapula-to-scapula scapulopexy, pre- and post-operation. The scapulas are tethered together into a retracted position with an Achilles tendon graft. In the right image, the rhomboid major muscles are distinguishable.
Prognosis
Genetics partially predicts prognosis.[citation needed] Those with fewer D4Z4 repeats, and those who have the genetics of both FSHD1 and FSHD2, are more likely to have severe disease.[citation needed] It has also been observed that disease manifestation is milder when a prominent family history is present, as opposed to a new mutation.[citation needed] In some large families, 30% of those with the mutation do not show symptoms, and 30% of those with symptoms do not progress beyond facial and shoulder weakness.[citation needed] Women are less likely to be symptomatic, and if symptomatic have less severe manifestations.[citation needed]
Epidemiology
The prevalence of FSHD ranges from 1 in 8,333 to 1 in 15,000.[2] The Netherlands reports a prevalence of 1 in 8,333, after accounting for the undiagnosed.[81] The prevalence in the United States is commonly quoted as 1 in 15,000.[11]
After genetic testing became possible in 1992, average prevalence was found to be around 1 in 20,000, a large increase compared to before 1992.[82][23][81] However, 1 in 20,000 is likely an underestimation, since many with FSHD have mild symptoms and are never diagnosed, or they are siblings of affected individuals and never seek definitive diagnosis.[81]
Race and ethnicity have not been shown to affect FSHD incidence or severity.[11]
Although the inheritance of FSHD shows no predilection for biological sex, the disease manifests less often in women, and even when it manifests in women, they on average are less severely affected than affected males.[11] Estrogen has been suspected to be a protective factor that accounts for this discrepancy. One study found that estrogen reduced DUX4 activity.[83] However, another study found no association between disease severity and lifetime estrogen exposure in females. The same study found that disease progression was not different through periods of hormonal changes, such as menarche, pregnancy, and menopause.[84]
History
The first description of a person with FSHD in medical literature appears in an autopsy report by Jean Cruveilhier in 1852.[12][13] In 1868, Duchenne published his seminal work on Duchenne muscular dystrophy, and as part of its differential was a description of FSHD.[85][13] First in 1874, then with a more commonly cited publication in 1884, and again with pictures in 1885, the French physicians Louis Landouzy and Joseph Dejerine published details of the disease, recognizing it as a distinct clinical entity, and thus FSHD is sometimes referred to as Landouzy Dejerine disease.[14][13] In their paper of 1886, Landouzy and Dejerine drew attention to the familial nature of the disorder and mentioned that four generations were affected in the kindred that they had investigated.[86] Formal definition of FSHD's clinical features did not occur until 1952 when a large Utah family with FSHD was studied. Beginning about 1980 an increasing interest in FSHD led to increased understanding of the great variability in the disease and a growing understanding of the genetic and pathophysiological complexities. By the late 1990s, researchers were finally beginning to understand the regions of chromosome 4 associated with FSHD.[36]
Since the publication of the unifying theory in 2010, researchers continued to refine their understanding of DUX4. With increasing confidence in this work, researchers proposed the first a consensus view in 2014 of the pathophysiology of the disease and potential approaches to therapeutic intervention based on that model.[21]
Over the years, FSHD has, at various times, been referred to as:
- facioscapulohumeral disease[17]
- faciohumeroscapular [citation needed]
- Landouzy-Dejerine disease[17]
- Landouzy-Dejerine syndrome[86]
- Landouzy-Dejerine type of muscular dystrophy[17]
- Erb-Landouzy-Dejerine syndrome[citation needed]
Chronology of important FSHD-related genetic research
| 1861 |  |
| 1884 | Landouzy and Dejerine describe a form of childhood progressive muscle atrophy with a characteristic involvement of facial muscles and distinct from pseudohypertrophic (Duchenne’s MD) and spinal muscle atrophy in adults.[87] |
| 1886 | Landouzy and Dejerine describe progressive muscular atrophy of the scapulo-humeral type.[88] |
| 1950 | Tyler and Stephens study 1249 individuals from a single kindred with FSHD traced to a single ancestor and describe a typical Mendelian inheritance pattern with complete penetrance and highly variable expression. The term facioscapulohumeral dystrophy is introduced.[89] |
| 1982 | Padberg provides the first linkage studies to determine the genetic locus for FSHD in his seminal thesis "Facioscapulohumeral disease."[17] |
| 1987 | The complete sequence of the Dystrophin gene (Duchenne’s MD) is determined.[90] |
| 1991 | The genetic defect in FSHD is linked to a region (4q35) near the tip of the long arm of chromosome 4.[91] |
| 1992 | FSHD, in both familial and de novo cases, is found to be linked to a recombination event that reduces the size of 4q EcoR1 fragment to < 28 kb (50–300 kb normally).[64] |
| 1993 | 4q EcoR1 fragments are found to contain tandem arrangement of multiple 3.3-kb units (D4Z4), and FSHD is associated with the presence of < 11 D4Z4 units.[63]
A study of seven families with FSHD reveals evidence of genetic heterogeneity in FSHD.[92] |
| 1994 | The heterochromatic structure of 4q35 is recognized as a factor that may affect the expression of FSHD, possibly via position-effect variegation.[93]
DNA sequencing within D4Z4 units shows they contain an open reading frame corresponding to two homeobox domains, but investigators conclude that D4Z4 is unlikely to code for a functional transcript.[93][94] |
| 1995 | The terms FSHD1A and FSHD1B are introduced to describe 4q-linked and non-4q-linked forms of the disease.[95] |
| 1996 | FSHD Region Gene1 (FRG1) is discovered 100 kb proximal to D4Z4.[96] |
| 1998 | Monozygotic twins with vastly different clinical expression of FSHD are described.[15] |
| 1999 | Complete sequencing of 4q35 D4Z4 units reveals a promoter region located 149 bp 5' from the open reading frame for the two homeobox domains, indicating a gene that encodes a protein of 391 amino acid protein (later corrected to 424 aa[97]), given the name DUX4.[98] |
| 2001 | Investigators assessed the methylation state (heterochromatin is more highly methylated than euchromatin) of DNA in 4q35 D4Z4. An examination of SmaI, MluI, SacII, and EagI restriction fragments from multiple cell types, including skeletal muscle, revealed no evidence for hypomethylation in cells from FSHD1 patients relative to D4Z4 from unaffected control cells or relative to homologous D4Z4 sites on chromosome 10. However, in all instances, D4Z4 from sperm was hypomethylated relative to D4Z4 from somatic tissues.[99] |
| 2002 | A polymorphic segment of 10 kb directly distal to D4Z4 is found to exist in two allelic forms, designated 4qA and 4qB. FSHD1 is associated solely with the 4qA allele.[100]
Three genes (FRG1, FRG2, ANT1) located in the region just centromeric to D4Z4 on chromosome 4 are found in isolated muscle cells from individuals with FSHD at levels 10 to 60 times greater than normal, showing a linkage between D4Z4 contractions and altered expression of 4q35 genes.[101] |
| 2003 | A further examination of DNA methylation in different 4q35 D4Z4 restriction fragments (BsaAI and FseI) showed significant hypomethylation at both sites for individuals with FSHD1, non-FSHD-expressing gene carriers, and individuals with phenotypic FSHD relative to unaffected controls.[102] |
| 2004 | Contraction of the D4Z4 region on the 4qB allele to < 38 kb does not cause FSHD.[103] |
| 2006 | Transgenic mice overexpressing FRG1 are shown to develop severe myopathy.[104] |
| 2007 | The DUX4 open reading frame is found to have been conserved in the genome of primates for over 100 million years, supporting the likelihood that it encodes a required protein.[105]
Researchers identify DUX4 mRNA in primary FSHD myoblasts and identify in D4Z4-transfected cells a DUX4 protein, the overexpression of which induces cell death.[97] DUX4 mRNA and protein expression are reported to increase in myoblasts from FSHD patients, compared to unaffected controls. Stable DUX4 mRNA is transcribed only from the most distal D4Z4 unit, which uses an intron and a polyadenylation signal provided by the flanking pLAM region. DUX4 protein is identified as a transcription factor, and evidence suggests overexpression of DUX4 is linked to an increase in the target paired-like homeodomain transcription factor 1 (PITX1).[106] |
| 2009 | The terms FSHD1 and FSHD2 are introduced to describe D4Z4-deletion-linked and non-D4Z4-deletion-linked genetic forms, respectively. In FSHD1, hypomethylation is restricted to the short 4q allele, whereas FSHD2 is characterized by hypomethylation of both 4q and both 10q alleles.[107]
Splicing and cleavage of the terminal (most telomeric) 4q D4Z4 DUX4 transcript in primary myoblasts and fibroblasts from FSHD patients is found to result in the generation of multiple RNAs, including small noncoding RNAs, antisense RNAs and capped mRNAs as new candidates for the pathophysiology of FSHD.[108] |
| 2010 | A unifying genetic model of FSHD is established: D4Z4 contractions only cause FSHD when in the context of a 4qA allele due to stabilization of DUX4 RNA transcript, allowing DUX4 expression.[7] Several organizations including The New York Times highlighted this research[109] (See FSHD Society).
Dr. Francis Collins, who oversaw the first sequencing of the Human Genome with the National Institutes of Health stated:[109]
Daniel Perez, co-founder of the FSHD Society, hailed the new findings saying:[citation needed]
The MDA stated that:[citation needed]
One of the report's co-authors, Silvère van der Maarel of the University of Leiden, stated that[citation needed]
DUX4 is found actively transcribed in skeletal muscle biopsies and primary myoblasts. FSHD-affected cells produce a full length transcript, DUX4-fl, whereas alternative splicing in unaffected individuals results in the production of a shorter, 3'-truncated transcript (DUX4-s). The low overall expression of both transcripts in muscle is attributed to relatively high expression in a small number of nuclei (~ 1 in 1000). Higher levels of DUX4 expression in human testis (~100 fold higher than skeletal muscle) suggest a developmental role for DUX4 in human development. Higher levels of DUX4-s (vs DUX4-fl) are shown to correlate with a greater degree of DUX-4 H3K9me3-methylation.[6] |
| 2012 | Some instances of FSHD2 are linked to mutations in the SMCHD1 gene on chromosome 18, and a genetic/mechanistic intersection of FSHD1 and FSHD2 is established.[50]
The prevalence of FSHD-like D4Z4 deletions on permissive alleles is significantly higher than the prevalence of FSHD in the general population, challenging the criteria for molecular diagnosis of FSHD.[110] When expressed in primary myoblasts, DUX4-fl acted as a transcriptional activator, producing a > 3-fold change in the expression of 710 genes.[111] A subsequent study using a larger number of samples identified DUX4-fl expression in myogenic cells and muscle tissue from unaffected relatives of FSHD patients, per se, is not sufficient to cause pathology, and that additional modifiers are determinants of disease progression.[112] Mechanism proposed of DBE-T (D4Z4 Regulatory Element transcript) leading to de-repression of 4q35 genes.[44] |
| 2013 | Mutations in SMCHD1 are shown to increase the severity of FSHD1.[54]
Transgenic mice carrying D4Z4 arrays from an FSHD1 allele (with 2.5 D4Z4 units), although lacking an obvious FSHD-like skeletal muscle phenotype, are found to recapitulate important genetic expression patterns and epigenetic features of FSHD.[113] |
| 2014 | DUX4-fl and downstream target genes are expressed in skeletal muscle biopsies and biopsy-derived cells of fetuses with FSHD-like D4Z4 arrays, indicating that molecular markers of FSHD are already expressed during fetal development.[114]
Researchers "review how the contributions from many labs over many years led to an understanding of a fundamentally new mechanism of human disease" and articulate how the unifying genetic model and subsequent research represent a "pivot-point in FSHD research, transitioning the field from discovery-oriented studies to translational studies aimed at developing therapies based on a sound model of disease pathophysiology." They describe the consensus mechanism of pathophysiology for FSHD as a "inefficient repeat-mediated epigenetic repression of the D4Z4 macrosatellite repeat array on chromosome 4, resulting in the variegated expression of the DUX4 retrogene, encoding a double-homeobox transcription factor, in skeletal muscle."[21] |
Past pharmaceutical development
Early drug trials, before the pathogenesis involving DUX4 was discovered, were untargeted and largely unsuccessful.[115] Most compounds were trialed on the basis of increasing muscle mass or decreasing inflammation.[115] Drugs that failed to show efficacy include:
- Prednisone, a steroid, was trialed due to its therapeutic effect in Duchenne muscular dystrophy.[116]
- Oral albuterol, a β2 agonist, although it improved muscle mass and certain measures of strength in clinical trials, it did not improve global strength or function.[117][118][119] Interestingly, after DUX4 was identified as an integral part of FSHD pathophysiology, drug screens showed that β2 agonists reduce DUX4 expression.[120]
- Diltiazem, a calcium channel blocker, was trialed in FSHD on the bases of anecdotal reports of it being beneficial and the theory that calcium dysregulation may play a part in muscle cell death (this was before identification of DUX4 as part of pathophysiology).[121]
- MYO-029 (Stamulumab) was developed to promote muscle growth. It is an antibody that inhibits myostatin, a protein that inhibits the growth of muscle tissue.[122]
- ACE-083 is a TGF-β inhibitor was developed to promote muscle growth.[123]
Society and culture
- In the Amazon Video series The Man in the High Castle, Obergruppenführer John Smith's son, Thomas, is diagnosed with Landouzy-Dejerine syndrome.
- In the biography Stuart: A Life Backwards, the protagonist was affected by FSHD.
- Chris Carrino, the radio voice of the Brooklyn Nets, is affected by FSHD. He created the Chris Carrino Foundation for FSHD.
FSHD Society
In 1991 the FSHD Society (named "FSH Society" until 2019)[124] was founded by two individuals with FSHD, Daniel Perez and Stephen Jacobsen. The FSHD Society raised funding to provide seed grants for FSHD research, advocated for the field to standardize the name of the disease as facioscapulohumeral muscular dystrophy and FSHD, and co-wrote the MD-CARE Act, passed into law in 2001, which for the first time mandated federal resources, including National Institutes of Health funding, for all muscular dystrophies. The FSHD Society has grown into the world's largest grassroots organization advocating for patient education and scientific and medical research.[125]
FSHD-EUROPE
In 2009 the FSHD-EUROPE was founded by European associations.[126]
Research directions
Pharmaceutical development
After achieving consensus on FSHD pathophysiology in 2014, researchers proposed four approaches for therapeutic intervention:[21]
- enhance the epigenetic repression of the D4Z4
- target the DUX4 mRNA, including altering splicing or polyadenylation;
- block the activity of the DUX4 protein
- inhibit the DUX4-induced process, or processes, that leads to pathology.
Small molecule drugs
Most drugs used in medicine are "small molecule drugs," as opposed to biologic medical products that include proteins, vaccines, and nucleic acids. Small molecule drugs can typically be taken by ingestion, rather than injection.
- Losmapimod, a selective inhibitor of p38α/β mitogen-activated protein kinases, was identified by Fulcrum Therapeutics as a potent suppressor of DUX4 in vitro.[128] A phase IIb clinical trial started in July 2019 and is expected to end in August 2020.[129]
- Casein kinase 1 (CK1) inhibition has been identified by Facio Therapies, a Dutch pharmaceutical company, to repress DUX4 expression and is in preclinical development. Facio Therapies claims that CK1 inhibition leaves myotube fusion intact, unlike BET inhibitors, p38 MAPK inhibitors, and β2 agonists.[130][131]
Gene therapy
Gene therapy is the administration of nucleotides to treat disease. Multiple types of gene therapy are in the preclinical stage of development for the treatment of FSHD.
- Antisense therapy utilizes antisense oligonucleotides that bind to DUX4 messenger RNA, inducing its degradation and preventing DUX4 protein production. Phosphorodiamidate morpholinos, an oligonucleotide modified to increase its stability, have been shown to selectively reduce DUX4 and its effects; however, these antisense nucleotides have poor ability to penetrate muscle.[2]
- MicroRNAs (miRNAs) directed against DUX4, delivered by viral vectors, have shown to reduce DUX4, protect against muscle pathology, and prevent loss of grip strength in mouse FSHD models.[2] Arrowhead pharmaceuticals is developing an RNA interference therapeutic against DUX4, named ARO-DUX4, and intends to file for regulatory clearance in third quarter of 2021 to begin clinical trials.[132][133]
- Genome editing, the permanent alteration of genetic code, is being researched. One study tried to use CRISPER-Cas9 to knockout the polyadenylation signal in lab dish models, but was unable to show therapeutic results.[134]
Potential drug targets
- Inhibition of the hyaluronic acid (HA) pathway is a potential therapy. One study found that many DUX4-induced molecular pathologies are mediated by HA signaling, and inhibition of HA biosynthesis with 4-methylumbelliferone prevented these molecular pathologies.[135]
- P300 inhibition has shown to inhibit the deleterious effects of DUX4[136]
- BET inhibitors have been shown to reduce DUX4 expression.[120]
- Antioxidants could potentially reduce the effects of FSHD. One study found that vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation increased endurance and strength of the quadriceps, but had no significant benefit on walking performance.[137] Further study is warranted.[2]
Outcome measures
Ways of measuring the disease are important for studying disease progression and assessing the efficacy of drugs in clinical trials.
- Quality of life can be measured with questionnaires, such as the FSHD Health Index.[138][2]
- How the disease affects daily activities can measured with questionnaires, such as the FSHD‐Rasch‐built overall disability scale (FSHD-RODS).[139]
- Electrical impedance myography is being studied as a way to measure muscle damage.[2]
- Muscle MRI is useful for assessment of all the muscles in the body. Muscles can be scored based on the degree of fat infiltration.[2]
References
- ^ "Facioscapulohumeral". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao Wagner, Kathryn R. (December 2019). "Facioscapulohumeral Muscular Dystrophies". CONTINUUM: Lifelong Learning in Neurology. 25 (6): 1662–1681. doi:10.1212/CON.0000000000000801. PMID 31794465. S2CID 208531681.
- ^ a b c d e f g h i j k l m n o p q r s t Mul, Karlien; Lassche, Saskia; Voermans, Nicol C; Padberg, George W; Horlings, Corinne GC; van Engelen, Baziel GM (June 2016). "What's in a name? The clinical features of facioscapulohumeral muscular dystrophy". Practical Neurology. 16 (3): 201–207. doi:10.1136/practneurol-2015-001353. PMID 26862222. S2CID 4481678.
- ^ a b Kumar, Vinay; Abbas, Abul; Aster, Jon, eds. (2018). Robbins Basic Pathology (Tenth ed.). Philadelphia, Pennsylvania: Elsevier. p. 844. ISBN 978-0-323-35317-5.
- ^ De Iaco, A; Planet, E; Coluccio, A; Verp, S; Duc, J; Trono, D (June 2017). "DUX-family transcription factors regulate zygotic genome activation in placental mammals". Nature Genetics. 49 (6): 941–945. doi:10.1038/ng.3858. PMC 5446900. PMID 28459456.
- ^ a b c Snider, L; Geng, LN; Lemmers, RJ; Kyba, M; Ware, CB; Nelson, AM; Tawil, R; Filippova, GN; van der Maarel, SM; Tapscott, SJ; Miller, DG (28 October 2010). "Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene". PLOS Genetics. 6 (10): e1001181. doi:10.1371/journal.pgen.1001181. PMC 2965761. PMID 21060811.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h i j k Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camaño P, Dauwerse JG, Snider L, Straasheijm KR, van Ommen GJ, Padberg GW, Miller DG, Tapscott SJ, Tawil R, Frants RR, van der Maarel SM (19 August 2010). "A Unifying Genetic Model for Facioscapulohumeral Muscular Dystrophy" (PDF). Science. 329 (5999): 1650–3. Bibcode:2010Sci...329.1650L. doi:10.1126/science.1189044. hdl:1887/117104. PMC 4677822. PMID 20724583. Archived from the original (PDF) on 2014-06-05.
- ^ Lemmers RJ, Wohlgemuth M, van der Gaag KJ, et al. (November 2007). "Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy". Am. J. Hum. Genet. 81 (5): 884–94. doi:10.1086/521986. PMC 2265642. PMID 17924332.
- ^ a b Eren, İlker; Birsel, Olgar; Öztop Çakmak, Özgür; Aslanger, Ayça; Gürsoy Özdemir, Yasemin; Eraslan, Serpil; Kayserili, Hülya; Oflazer, Piraye; Demirhan, Mehmet (May 2020). "A novel shoulder disability staging system for scapulothoracic arthrodesis in patients with facioscapulohumeral dystrophy". Orthopaedics & Traumatology: Surgery & Research. 106 (4): 701–707. doi:10.1016/j.otsr.2020.03.002. PMID 32430271.
- ^ a b c d e f g Tawil, R; Van Der Maarel, SM (July 2006). "Facioscapulohumeral muscular dystrophy" (PDF). Muscle & Nerve. 34 (1): 1–15. doi:10.1002/mus.20522. PMID 16508966. S2CID 43304086.
- ^ a b c d Statland, JM; Tawil, R (December 2016). "Facioscapulohumeral Muscular Dystrophy". Continuum (Minneapolis, Minn.). 22 (6, Muscle and Neuromuscular Junction Disorders): 1916–1931. doi:10.1212/CON.0000000000000399. PMC 5898965. PMID 27922500.
- ^ a b Cruveilhiers, J. (1852–1853). "Mémoire sur la paralysie musculaire atrophique". Bulletins de l'Académie de Médecine. 18: 490–502, 546–583.
- ^ a b c d Rogers, Mark T. (2004). "Facioscapulohumeral muscular dystrophy: historical bacground and literature review". In Upadhyaya, Meena; Cooper, David N. (eds.). FSHD facioscapulohumeral muscular dystrophy : clinical medicine and molecular cell biology. BIOS Scientific Publishers. ISBN 1-85996-244-0.
- ^ a b Landouzy, L.; Dejerine, J. (1885). Landouzy, L.; Lépine, R. (eds.). "De la myopathie atrophique progressive (myopathie sans neuropathie débutant d'ordinaire dans l'enfance par la face)". Revue de Médecine (in French). 5. Felix Alcan: 253–366. Retrieved 19 May 2020.
- ^ a b Tupler, R; Barbierato, L; et al. (Sep 1998). "Identical de novo mutation at the D4F104S1 locus in monozygotic male twins affected by facioscapulohumeral muscular dystrophy (FSHD) with different clinical expression". Journal of Medical Genetics. 35 (9): 778–783. doi:10.1136/jmg.35.9.778. PMC 1051435. PMID 9733041.
- ^ Tawil, R; Storvick, D; Feasby, TE; Weiffenbach, B; Griggs, RC (February 1993). "Extreme variability of expression in monozygotic twins with FSH muscular dystrophy". Neurology. 43 (2): 345–8. doi:10.1212/wnl.43.2.345. PMID 8094896. S2CID 44422140.
- ^ a b c d e Padberg, GW (1982-10-13). Facioscapulohumeral disease (Thesis). Leiden University.
- ^ a b c Rijken, NH; van der Kooi, EL; Hendriks, JC; van Asseldonk, RJ; Padberg, GW; Geurts, AC; van Engelen, BG (December 2014). "Skeletal muscle imaging in facioscapulohumeral muscular dystrophy, pattern and asymmetry of individual muscle involvement". Neuromuscular Disorders. 24 (12): 1087–96. doi:10.1016/j.nmd.2014.05.012. PMID 25176503. S2CID 101093.
- ^ Pandya, Shree; Eichinger, Kate. "Physical Therapy for Facioscapulohumeral Muscular Dystrophy" (PDF). FSHD Society. Archived from the original (PDF) on 14 April 2020. Retrieved 14 April 2020.
- ^ a b c Upadhyaya, Meena; Cooper, David, eds. (March 2004). FSHD Facioscapulohumeral Muscular Dystrophy : Clinical Medicine and Molecular Cell Biology. BIOS Scientific Publishers. ISBN 0203483677.
- ^ a b c d e f g h i j k l m Tawil, Rabi; van der Maarel, SM; Tapscott, SJ (10 June 2014). "Facioscapulohumeral dystrophy: the path to consensus on pathophysiology". Skeletal Muscle. 4 (1): 12. doi:10.1186/2044-5040-4-12. PMC 4060068. PMID 24940479.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Trevisan, CP; Pastorello, E; Tomelleri, G; Vercelli, L; Bruno, C; Scapolan, S; Siciliano, G; Comacchio, F (December 2008). "Facioscapulohumeral muscular dystrophy: hearing loss and other atypical features of patients with large 4q35 deletions". European Journal of Neurology. 15 (12): 1353–8. doi:10.1111/j.1468-1331.2008.02314.x. PMID 19049553. S2CID 26276887.
- ^ a b c d e Padberg, George W. (2004). "Facioscapulohumeral muscular dystrophy: a clinician's experience". In Upadhyaya, Meena; Cooper, David N. (eds.). FSHD Facioscapulohumeral Muscular Dystrophy: Clinical Medicine and Molecular Cell Biology. BIOS Scientific Publishers. ISBN 1-85996-244-0.
- ^ a b "A giant of FSHD research shares his "regrets"". FSHD Society. Way Back Machine. 30 September 2020. Archived from the original on 24 October 2020. Retrieved 11 March 2021.
Another striking aspect of FSHD is that muscles weakness seems to vary so much from patient to patient. Nonetheless, "there is a highly characteristic pattern of muscle weakness, otherwise we would never have been able to recognize FSHD as a specific disease," Padberg said. "Strong deltoid muscle does not occur in any other condition that involves weakness of scapular stabilizers. No other muscle disease with shoulder girdle involvement has this pattern." Unfortunately, "an explanation is beyond our grasp as we don't know how muscle are laid down" during the early stages of human gestation.
- ^ Pandya, Shree; King, Wendy M; Tawil, Rabi (1 January 2008). "Facioscapulohumeral Dystrophy". Physical Therapy. 88 (1): 105–113. doi:10.2522/ptj.20070104. PMID 17986494.
- ^ a b c Tasca, G; Monforte, M; Iannaccone, E; Laschena, F; Ottaviani, P; Leoncini, E; Boccia, S; Galluzzi, G; Pelliccioni, M; Masciullo, M; Frusciante, R; Mercuri, E; Ricci, E (2014). "Upper girdle imaging in facioscapulohumeral muscular dystrophy". PLOS ONE. 9 (6): e100292. Bibcode:2014PLoSO...9j0292T. doi:10.1371/journal.pone.0100292. PMC 4059711. PMID 24932477.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Gerevini, S; Scarlato, M; Maggi, L; Cava, M; Caliendo, G; Pasanisi, B; Falini, A; Previtali, SC; Morandi, L (March 2016). "Muscle MRI findings in facioscapulohumeral muscular dystrophy". European Radiology. 26 (3): 693–705. doi:10.1007/s00330-015-3890-1. PMID 26115655. S2CID 24650482.
- ^ a b c Mair, D; Huegens-Penzel, M; Kress, W; Roth, C; Ferbert, A (2017). "Leg Muscle Involvement in Facioscapulohumeral Muscular Dystrophy: Comparison between Facioscapulohumeral Muscular Dystrophy Types 1 and 2". European Neurology. 77 (1–2): 32–39. doi:10.1159/000452763. PMID 27855411. S2CID 25005883.
- ^ a b c Olsen, DB; Gideon, P; Jeppesen, TD; Vissing, J (November 2006). "Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI". Journal of Neurology. 253 (11): 1437–41. doi:10.1007/s00415-006-0230-z. PMID 16773269. S2CID 19421344.
- ^ Padberg, G. W.; Brouwer, O. F.; de Keizer, R. J. W.; Dijkman, G.; Wijmenga, C.; Grote, J. J.; Frants, R. R. (1995). "On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy". Muscle & Nerve. 18 (S13): S73–S80. doi:10.1002/mus.880181314. hdl:2066/20764.
- ^ Lindner, Moritz; Holz, Frank G; Charbel Issa, Peter (2016-04-27). "Spontaneous resolution of retinal vascular abnormalities and macular oedema in facioscapulohumeral muscular dystrophy". Clinical & Experimental Ophthalmology. 44 (7): 627–628. doi:10.1111/ceo.12735. ISSN 1442-6404. PMID 26933772. S2CID 204996841.
- ^ a b Eren, İ; Abay, B; Günerbüyük, C; Çakmak, ÖÖ; Şar, C; Demirhan, M (February 2020). "Spinal fusion in facioscapulohumeral dystrophy for hyperlordosis: A case report". Medicine. 99 (8): e18787. doi:10.1097/MD.0000000000018787. PMID 32080072.
- ^ Huml, Raymond A.; Perez, Daniel P. (2015). "FSHD: The Most Common Type of Muscular Dystrophy?". Muscular Dystrophy: 9–19. doi:doi.org/10.1007/978-3-319-17362-7_3.
{{cite journal}}: Check|doi=value (help) - ^ Wohlgemuth M, van der Kooi EL, van Kesteren RG, van der Maarel SM, Padberg GW (2004). "Ventilatory support in facioscapulohumeral muscular dystrophy". Neurology. 63 (1): 176–8. CiteSeerX 10.1.1.543.2968. doi:10.1212/01.wnl.0000133126.86377.e8. PMID 15249635. S2CID 31335126.
- ^ a b c d e f g Lemmers, Richard J.L.F.; O’Shea, Suzanne; Padberg, George W.; Lunt, Peter W.; van der Maarel, Silvère M. (May 2012). "Best practice guidelines on genetic diagnostics of Facioscapulohumeral muscular dystrophy: Workshop 9th June 2010, LUMC, Leiden, The Netherlands". Neuromuscular Disorders. 22 (5): 463–470. doi:10.1016/j.nmd.2011.09.004. PMID 22177830. S2CID 39898514.
- ^ a b c d Impossible Things: Through the looking glass with FSH Dystrophy Researchers, Margaret Wahl, MDA, Quest magazine, Vol 14, No 2, March–April 2007
- ^ Dixit M, Ansseau E, Tassin A, et al. (November 2007). "DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1". Proc. Natl. Acad. Sci. U.S.A. 104 (46): 18157–62. Bibcode:2007PNAS..10418157D. doi:10.1073/pnas.0708659104. PMC 2084313. PMID 17984056.
- ^ White, J.A.; McAlpine, P.J.; Antonarakis, S.; Cann, H.; Eppig, J.T.; Frazer, K.; Frezal, J.; Lancet, D.; Nahmias, J.; Pearson, P.; Peters, J.; Scott, A.; Scott, H.; Spurr, N.; Talbot, C.; Povey, S. (October 1997). "NOMENCLATURE". Genomics. 45 (2): 468–471. doi:10.1006/geno.1997.4979. PMID 9344684.
- ^ Fasman, KH; Letovsky, SI; Cottingham, RW; Kingsbury, DT (1 January 1996). "Improvements to the GDB Human Genome Data Base". Nucleic Acids Research. 24 (1): 57–63. doi:10.1093/nar/24.1.57. PMC 145602. PMID 8594601.
- ^ Coppée, Frédérique; Mattéotti, Christel; Anssaeu, Eugénie; Sauvage, Sébastien; Leclercq, India; Leroy, Axelle; Marcowycz, Aline; Gerbaux, Cécile; Figlewicz, Denise; Ding, Hao; Belayew, Belayew (2004). "The DUX gene family and FSHD". In Upadhyaya, Meena; Cooper, David N. (eds.). FSHD facioscapulohumeral muscular dystrophy : clinical medicine and molecular cell biology. BIOS Scientific Publishers. ISBN 1-85996-244-0.
- ^ Rossi M, Ricci E, Colantoni L, et al. (2007). "The Facioscapulohumeral muscular dystrophy region on 4qter and the homologous locus on 10qter evolved independently under different evolutionary pressure". BMC Med. Genet. 8: 8. doi:10.1186/1471-2350-8-8. PMC 1821008. PMID 17335567.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lemmers, RJLF; van der Vliet, PJ; Blatnik, A; Balog, J; Zidar, J; Henderson, D; Goselink, R; Tapscott, SJ; Voermans, NC; Tawil, R; Padberg, GWAM; van Engelen, BG; van der Maarel, SM (12 January 2021). "Chromosome 10q-linked FSHD identifies DUX4 as principal disease gene". Journal of Medical Genetics: jmedgenet-2020-107041. doi:10.1136/jmedgenet-2020-107041. PMID 33436523. S2CID 231589589.
- ^ Himeda, CL; Jones, PL (31 August 2019). "The Genetics and Epigenetics of Facioscapulohumeral Muscular Dystrophy". Annual Review of Genomics and Human Genetics. 20: 265–291. doi:10.1146/annurev-genom-083118-014933. PMID 31018108.
- ^ a b Cabianca, DS; Casa, Casa; Bodega, B; et al. (May 11, 2012). "A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy". Cell. 149 (4): 819–831. doi:10.1016/j.cell.2012.03.035. PMC 3350859. PMID 22541069.
- ^ a b c d e f g Sacconi, S; Briand-Suleau, A; Gros, M; Baudoin, C; Lemmers, RJLF; Rondeau, S; Lagha, N; Nigumann, P; Cambieri, C; Puma, A; Chapon, F; Stojkovic, T; Vial, C; Bouhour, F; Cao, M; Pegoraro, E; Petiot, P; Behin, A; Marc, B; Eymard, B; Echaniz-Laguna, A; Laforet, P; Salviati, L; Jeanpierre, M; Cristofari, G; van der Maarel, SM (7 May 2019). "FSHD1 and FSHD2 form a disease continuum". Neurology. 92 (19): e2273–e2285. doi:10.1212/WNL.0000000000007456. PMC 6537132. PMID 30979860.
- ^ Tupler, R; Berardinelli, A; Barbierato, L; Frants, R; Hewitt, JE; Lanzi, G; Maraschio, P; Tiepolo, L (May 1996). "Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy". Journal of Medical Genetics. 33 (5): 366–70. doi:10.1136/jmg.33.5.366. PMC 1050603. PMID 8733044.
- ^ Tawil, R; Forrester, J; Griggs, RC; Mendell, J; Kissel, J; McDermott, M; King, W; Weiffenbach, B; Figlewicz, D (June 1996). "Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. The FSH-DY Group". Annals of Neurology. 39 (6): 744–8. doi:10.1002/ana.410390610. PMID 8651646. S2CID 84518968.
- ^ Zernov, N; Skoblov, M (13 March 2019). "Genotype-phenotype correlations in FSHD". BMC Medical Genomics. 12 (Suppl 2): 43. doi:10.1186/s12920-019-0488-5. PMC 6416831. PMID 30871534.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sacconi, S; Salviati, L; Desnuelle, C (April 2015). "Facioscapulohumeral muscular dystrophy". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1852 (4): 607–14. doi:10.1016/j.bbadis.2014.05.021. PMID 24882751.
- ^ a b c Lemmers, RJ; Tawil, R; Petek, LM; et al. (Dec 2012). "Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2". Nature Genetics. 44 (12): 1370–1374. doi:10.1038/ng.2454. PMC 3671095. PMID 23143600.
- ^ van den Boogaard, ML; Lemmers, RJLF; Balog, J; Wohlgemuth, M; Auranen, M; Mitsuhashi, S; van der Vliet, PJ; Straasheijm, KR; van den Akker, RFP; Kriek, M; Laurense-Bik, MEY; Raz, V; van Ostaijen-Ten Dam, MM; Hansson, KBM; van der Kooi, EL; Kiuru-Enari, S; Udd, B; van Tol, MJD; Nishino, I; Tawil, R; Tapscott, SJ; van Engelen, BGM; van der Maarel, SM (5 May 2016). "Mutations in DNMT3B Modify Epigenetic Repression of the D4Z4 Repeat and the Penetrance of Facioscapulohumeral Dystrophy". American Journal of Human Genetics. 98 (5): 1020–1029. doi:10.1016/j.ajhg.2016.03.013. PMC 4863565. PMID 27153398.
- ^ a b Johnson, NE; Statland, JM (7 May 2019). "FSHD1 or FSHD2: That is the question: The answer: It's all just FSHD". Neurology. 92 (19): 881–882. doi:10.1212/WNL.0000000000007446. PMID 30979855. S2CID 111390628.
- ^ a b c Hamanaka, Kohei; Šikrová, Darina; Mitsuhashi, Satomi; Masuda, Hiroki; Sekiguchi, Yukari; Sugiyama, Atsuhiko; Shibuya, Kazumoto; Lemmers, Richard J.L.F.; Goossens, Remko; Ogawa, Megumu; Nagao, Koji; Obuse, Chikashi; Noguchi, Satoru; Hayashi, Yukiko K.; Kuwabara, Satoshi; Balog, Judit; Nishino, Ichizo; van der Maarel, Silvère M. (28 May 2020). "Homozygous nonsense variant in associated with facioscapulohumeral muscular dystrophy". Neurology. 94 (23): e2441–e2447. doi:10.1212/WNL.0000000000009617. PMC 7455367. PMID 32467133. S2CID 218982743.
- ^ a b Sacconi, S; Lemmers, RJ; Balog, J; et al. (Oct 3, 2013). "The FSHD2 gene SMCHD1 is a modifier of disease severity in families affected by FSHD1". The American Journal of Human Genetics. 93 (4): 744–751. doi:10.1016/j.ajhg.2013.08.004. PMC 3791262. PMID 24075187.
- ^ a b Lim, KRQ; Nguyen, Q; Yokota, T (22 January 2020). "DUX4 Signalling in the Pathogenesis of Facioscapulohumeral Muscular Dystrophy". International Journal of Molecular Sciences. 21 (3): 729. doi:10.3390/ijms21030729. PMC 7037115. PMID 31979100.
- ^ a b Bosnakovski, Darko; Shams, Ahmed S.; Yuan, Ce; da Silva, Meiricris T.; Ener, Elizabeth T.; Baumann, Cory W.; Lindsay, Angus J.; Verma, Mayank; Asakura, Atsushi; Lowe, Dawn A.; Kyba, Michael (6 April 2020). "Transcriptional and cytopathological hallmarks of FSHD in chronic DUX4-expressing mice". Journal of Clinical Investigation. 130 (5): 2465–2477. doi:10.1172/JCI133303. PMC 7190912. PMID 32250341.
- ^ Lek, Angela; Zhang, Yuanfan; Woodman, Keryn G.; Huang, Shushu; DeSimone, Alec M.; Cohen, Justin; Ho, Vincent; Conner, James; Mead, Lillian; Kodani, Andrew; Pakula, Anna; Sanjana, Neville; King, Oliver D.; Jones, Peter L.; Wagner, Kathryn R.; Lek, Monkol; Kunkel, Louis M. (25 March 2020). "Applying genome-wide CRISPR-Cas9 screens for therapeutic discovery in facioscapulohumeral muscular dystrophy". Science Translational Medicine. 12 (536): eaay0271. doi:10.1126/scitranslmed.aay0271. PMC 7304480. PMID 32213627.
- ^ Wang, LH; Friedman, SD; Shaw, D; Snider, L; Wong, CJ; Budech, CB; Poliachik, SL; Gove, NE; Lewis, LM; Campbell, AE; Lemmers, RJFL; Maarel, SM; Tapscott, SJ; Tawil, RN (2019-02-01). "MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD". Human molecular genetics. 28 (3): 476–486. doi:10.1093/hmg/ddy364. PMC 6337697. PMID 30312408.
- ^ Strafella, Claudia; Caputo, Valerio; Galota, Rosaria Maria; Campoli, Giulia; Bax, Cristina; Colantoni, Luca; Minozzi, Giulietta; Orsini, Chiara; Politano, Luisa; Tasca, Giorgio; Novelli, Giuseppe; Ricci, Enzo; Giardina, Emiliano; Cascella, Raffaella (1 December 2019). "The variability of SMCHD1 gene in FSHD patients: evidence of new mutations". Human Molecular Genetics. 28 (23): 3912–3920. doi:10.1093/hmg/ddz239. PMC 6969370. PMID 31600781.
- ^ Zampatti, S; Colantoni, L; Strafella, C; Galota, RM; Caputo, V; Campoli, G; Pagliaroli, G; Carboni, S; Mela, J; Peconi, C; Gambardella, S; Cascella, R; Giardina, E (May 2019). "Facioscapulohumeral muscular dystrophy (FSHD) molecular diagnosis: from traditional technology to the NGS era". Neurogenetics. 20 (2): 57–64. doi:10.1007/s10048-019-00575-4. PMID 30911870. S2CID 85495566.
- ^ Kinoshita, June (11 March 2020). "Genetic testing for FSHD—a new frontier". FSHD Society. Archived from the original on 8 April 2020. Retrieved 8 April 2020.
- ^ Vasale, J; Boyar, F; Jocson, M; Sulcova, V; Chan, P; Liaquat, K; Hoffman, C; Meservey, M; Chang, I; Tsao, D; Hensley, K; Liu, Y; Owen, R; Braastad, C; Sun, W; Walrafen, P; Komatsu, J; Wang, JC; Bensimon, A; Anguiano, A; Jaremko, M; Wang, Z; Batish, S; Strom, C; Higgins, J (December 2015). "Molecular combing compared to Southern blot for measuring D4Z4 contractions in FSHD". Neuromuscular Disorders. 25 (12): 945–51. doi:10.1016/j.nmd.2015.08.008. PMID 26420234. S2CID 6871094.
- ^ a b van Deutekom, JC; Wijmenga, C; van Tienhoven, EA; et al. (Dec 1993). "FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit". Human Molecular Genetics. 2 (12): 2037–2042. doi:10.1093/hmg/2.12.2037. PMID 8111371.
- ^ a b Wijmenga, C; Hewitt, JE; Sandkuijl, LA; et al. (Sep 1992). "Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy". Nature Genetics. 2 (1): 26–30. doi:10.1038/ng0992-26. PMID 1363881. S2CID 21940164.
- ^ Frants, Rune R.; Sandkuijl, Lodewijk A.; van der Maarel, Silvere M.; Padberg, George W. (2004). "Mapping of the FSHD gene and the discovery of the pathognomonic deletion". In Upadhyaya, Meena; Cooper, David N. (eds.). FSHD Facioscapulohumeral Muscular Dystrophy: Clinical Medicine and Molecular Cell Biology. BIOS Scientific Publishers. ISBN 1-85996-244-0.
- ^ FSHD Fact Sheet Archived 2006-03-06 at the Wayback Machine, MDA, 11/1/2001
- ^ a b c Tawil, R; van der Maarel, S; Padberg, GW; van Engelen, BG (July 2010). "171st ENMC international workshop: Standards of care and management of facioscapulohumeral muscular dystrophy". Neuromuscular Disorders. 20 (7): 471–5. doi:10.1016/j.nmd.2010.04.007. PMID 20554202. S2CID 18448196.
- ^ a b Voet, N; Bleijenberg, G; Hendriks, J; de Groot, I; Padberg, G; van Engelen, B; Geurts, A (18 November 2014). "Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT". Neurology. 83 (21): 1914–22. doi:10.1212/WNL.0000000000001008. PMID 25339206. S2CID 25382403.
- ^ a b Janssen, B; Voet, N; Geurts, A; van Engelen, B; Heerschap, A (3 May 2016). "Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients". Neurology. 86 (18): 1700–7. doi:10.1212/WNL.0000000000002640. PMID 27037227. S2CID 11617226.
- ^ Voet, Nicoline Bm; van der Kooi, Elly L.; van Engelen, Baziel Gm; Geurts, Alexander Ch (6 December 2019). "Strength training and aerobic exercise training for muscle disease". The Cochrane Database of Systematic Reviews. 12: CD003907. doi:10.1002/14651858.CD003907.pub5. ISSN 1469-493X. PMC 6953420. PMID 31808555.
- ^ a b Tawil, R; Mah, JK; Baker, S; Wagner, KR; Ryan, MM; Sydney Workshop, Participants. (July 2016). "Clinical practice considerations in facioscapulohumeral muscular dystrophy Sydney, Australia, 21 September 2015". Neuromuscular Disorders. 26 (7): 462–71. doi:10.1016/j.nmd.2016.03.007. PMID 27185458.
- ^ "Information for Patients and Families - The Richard Fields Center for FSH Dystrophy (FSHD) & Neuromuscular Research - University of Rochester Medical Center". www.urmc.rochester.edu. Archived from the original on 14 November 2019. Retrieved 14 April 2020.
- ^ Aprile, I; Bordieri, C; Gilardi, A; Lainieri Milazzo, M; Russo, G; De Santis, F; Frusciante, R; Iannaccone, E; Erra, C; Ricci, E; Padua, L (April 2013). "Balance and walking involvement in facioscapulohumeral dystrophy: a pilot study on the effects of custom lower limb orthoses". European Journal of Physical and Rehabilitation Medicine. 49 (2): 169–78. PMID 23138679.
- ^ Sansone, V; Boynton, J; Palenski, C (June 1997). "Use of gold weights to correct lagophthalmos in neuromuscular disease". Neurology. 48 (6): 1500–3. doi:10.1212/wnl.48.6.1500. hdl:2434/210652. PMID 9191754. S2CID 16251273.
- ^ Matsumoto, M; Onoda, S; Uehara, H; Miura, Y; Katayama, Y; Kimata, Y (September 2016). "Correction of the Lower Lip With a Cartilage Graft and Lip Resection in Patients With Facioscapulohumeral Muscular Dystrophy". The Journal of Craniofacial Surgery. 27 (6): 1427–9. doi:10.1097/SCS.0000000000002720. PMID 27300465. S2CID 16343571.
- ^ Krishnamurthy, S; Ibrahim, M (January 2019). "Tendon Transfers in Foot Drop". Indian Journal of Plastic Surgery. 52 (1): 100–108. doi:10.1055/s-0039-1688105. PMC 6664842. PMID 31456618.
- ^ Chiodo, Chris; Bluman, Eric M. (2011-10-21). Tendon transfers in the foot and ankle. Saunders. p. 421. ISBN 9781455709243. Retrieved 1 January 2020.
- ^ Demirhan, Mehmet; Uysal, Ozgur; Atalar, Ata Can; Kilicoglu, Onder; Serdaroglu, Piraye (31 March 2009). "Scapulothoracic Arthrodesis in Facioscapulohumeral Dystrophy with Multifilament Cable". Clinical Orthopaedics and Related Research. 467 (8): 2090–2097. doi:10.1007/s11999-009-0815-9. PMC 2706357. PMID 19333668.
- ^ DeFranco, Michael J.; Nho, Shane; Romeo, Anthony A. (April 2010). "Scapulothoracic Fusion". Journal of the American Academy of Orthopaedic Surgeons. 18 (4): 236–42. doi:10.5435/00124635-201004000-00006. PMID 20357232. S2CID 27456684.
- ^ Abrams, Jeffrey S.; Bell, Robert H.; Tokish, John M. (2018). ADVANCED RECONSTRUCTION OF SHOULDER. AMER ACAD OF ORTHOPAEDIC. ISBN 9781975123475.
- ^ a b c Deenen JC, Arnts H, van der Maarel SM, Padberg GW, Verschuuren JJ, Bakker E, Weinreich SS, Verbeek AL, van Engelen BG (2014). "Population-based incidence and prevalence of facioscapulohumeral dystrophy". Neurology. 83 (12): 1056–9. doi:10.1212/WNL.0000000000000797. PMC 4166358. PMID 25122204.
- ^ Deenen, JC; Horlings, CG; Verschuuren, JJ; Verbeek, AL; van Engelen, BG (2015). "The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature". Journal of Neuromuscular Diseases. 2 (1): 73–85. doi:10.3233/JND-140045. PMID 28198707.
- ^ Teveroni, E; Pellegrino, M; Sacconi, S; Calandra, P; Cascino, I; Farioli-Vecchioli, S; Puma, A; Garibaldi, M; Morosetti, R; Tasca, G; Ricci, E; Trevisan, CP; Galluzzi, G; Pontecorvi, A; Crescenzi, M; Deidda, G; Moretti, F (3 April 2017). "Estrogens enhance myoblast differentiation in facioscapulohumeral muscular dystrophy by antagonizing DUX4 activity". The Journal of Clinical Investigation. 127 (4): 1531–1545. doi:10.1172/JCI89401. PMC 5373881. PMID 28263188.
- ^ Mul, K; Horlings, CGC; Voermans, NC; Schreuder, THA; van Engelen, BGM (June 2018). "Lifetime endogenous estrogen exposure and disease severity in female patients with facioscapulohumeral muscular dystrophy". Neuromuscular Disorders. 28 (6): 508–511. doi:10.1016/j.nmd.2018.02.012. PMID 29655530.
- ^ Duchenne, Guillaume-Benjamin (1868). "De la paralysie musculaire pseudo-hypertrophique, ou paralysie myo-sclérosique". Arch. Gen. Med. (in French). 11. Bibliothèque nationale de France: 5–25, 179–209, 305–321, 421–443, 552–588. Retrieved 18 May 2020.
- ^ a b Landouzy-Dejerine syndrome, whonamedit.com, date accessed March 11, 2007
- ^ Landouzy; Dejerine (1884). "De la myopathie atrophique progressive (myopathie héréditaire, débutant dans l'enfance par la face, sans altération du système nerveux)". Comptes Rendus de l'Académie des Sciences. 98: 53–55.
- ^ Landouzy; Dejerine (1886). "Contribution à l'étude de la myopathie atrophique progressive (myopathie atrophique progressive, à type scapulo-huméral)". Comptes Rendus des Séances de la Société de Biologie. 38: 478–481.
- ^ Tyler, Frank; Stephens, FE (April 1950). "Studies in disorders of muscle. II Clinical manifestations and inheritance of facioscapulohumeral dystrophy in a large family". Annals of Internal Medicine. 32 (4): 640–660. doi:10.7326/0003-4819-32-4-640. PMID 15411118.
- ^ Koenig, M; Hoffman, EP; Bertelson, CJ; Monaco, AP; Feener, C; Kunkel, LM (Jul 31, 1987). "Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals". Cell. 50 (3): 509–517. doi:10.1016/0092-8674(87)90504-6. PMID 3607877. S2CID 35668717.
- ^ Wijmenga, C; Padberg, GW; Moerer, P; et al. (April 1991). "Mapping of facioscapulohumeral muscular dystrophy gene to chromosome 4q35-qter by multipoint linkage analysis and in situ hybridization". Genomics. 9 (4): 570–575. doi:10.1016/0888-7543(91)90348-I. PMID 2037288.
- ^ Gilbert, JR; Stajich, JM; Wall, S; et al. (Aug 1993). "Evidence for heterogeneity in facioscapulohumeral muscular dystrophy (FSHD)". American Journal of Human Genetics. 53 (2): 401–408. PMC 1682358. PMID 8328457.
- ^ a b Winokur, ST; Bengtsson, U; Feddersen, J; et al. (May 1994). "The DNA rearrangement associated with facioscapulohumeral muscular dystrophy involves a heterochromatin-associated repetitive element: implications for a role of chromatin structure in the pathogenesis of the disease". Chromosome Research. 2 (3): 225–234. doi:10.1007/bf01553323. PMID 8069466. S2CID 6933736.
- ^ Hewitt, JE; Lyle, R; Clark, LN; et al. (Aug 1994). "Analysis of the tandem repeat locus D4Z4 associated with facioscapulohumeral muscular dystrophy". Human Molecular Genetics. 3 (8): 1287–1295. doi:10.1093/hmg/3.8.1287. PMID 7987304.
- ^ Gilbert, JR; Speer, MC; Stajich, J; et al. (Oct 1995). "Exclusion mapping of chromosomal regions which cross hybridise to FSHD1A associated markers in FSHD1B". Journal of Medical Genetics. 32 (10): 770–773. doi:10.1136/jmg.32.10.770. PMC 1051697. PMID 8558552.
- ^ van Deutekom, JC; Lemmers, RJ; Grewal, PK; et al. (May 1996). "Identification of the first gene (FRG1) from the FSHD region on human chromosome 4q35". Human Molecular Genetics. 5 (5): 581–590. doi:10.1093/hmg/5.5.581. PMID 8733123.
- ^ a b Kowaljow, V; Marcowycz, A; Ansseau, E; et al. (Aug 2007). "The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein". Neuromuscular Disorders. 17 (8): 611–623. doi:10.1016/j.nmd.2007.04.002. PMID 17588759. S2CID 25926418.
- ^ Gabriels, J; Beckers, MC; Ding, H; et al. (Aug 5, 1999). "Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element". Gene. 236 (1): 25–32. doi:10.1016/S0378-1119(99)00267-X. PMID 10433963.
- ^ Tsien, F; Sun, B; Hopkins, NE; et al. (Nov 2001). "Methylation of the FSHD syndrome-linked subtelomeric repeat in normal and FSHD cell cultures and tissues". Molecular Genetics and Metabolism. 74 (3): 322–331. doi:10.1006/mgme.2001.3219. PMID 11708861.
- ^ Lemmers, RJ; de Kievit, P; Sandkuijl, L; et al. (Oct 2002). "Facioscapulohumeral muscular dystrophy is uniquely associated with one of the two variants of the 4q subtelomere". Nature Genetics. 32 (2): 235–236. doi:10.1038/ng999. PMID 12355084. S2CID 28107557.
- ^ Gabellini, D; Green, MR; Tupler, R (Aug 9, 2002). "Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle". Cell. 110 (3): 339–348. doi:10.1016/S0092-8674(02)00826-7. PMID 12176321. S2CID 16396883.
- ^ van Overveld, PG; Lemmers, RJ; Sandkuijl, LA; et al. (Dec 2003). "Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy". Nature Genetics. 35 (4): 315–317. doi:10.1038/ng1262. PMID 14634647. S2CID 28696708.
- ^ Lemmers, RJ; Wohlgemuth, M; Frants, RR; Padberg, GW; Morava, E; van der Maarel, SM (Dec 2004). "Contractions of D4Z4 on 4qB subtelomeres do not cause facioscapulohumeral muscular dystrophy". The American Journal of Human Genetics. 75 (6): 1124–1130. doi:10.1086/426035. PMC 1182148. PMID 15467981.
- ^ Gabellini, D; D'Antona, G; Moggio, M; et al. (Feb 23, 2006). "Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1". Nature. 439 (7079): 973–977. Bibcode:2006Natur.439..973G. doi:10.1038/nature04422. PMID 16341202. S2CID 4427465.
- ^ Clapp, J; Mitchell, LM; Bolland, DJ; et al. (Aug 2007). "Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy". The American Journal of Human Genetics. 81 (2): 264–279. doi:10.1086/519311. PMC 1950813. PMID 17668377.
- ^ Dixit, M; Ansseau, E; Tassin, A; et al. (Nov 13, 2007). "DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1". Proceedings of the National Academy of Sciences of the USA. 104 (46): 18157–18162. Bibcode:2007PNAS..10418157D. doi:10.1073/pnas.0708659104. PMC 2084313. PMID 17984056.
- ^ de Greef, JC; Lemmers, RJ; van Engelen, BG; et al. (Oct 2009). "Common epigenetic changes of D4Z4 in contraction-dependent and contraction-independent FSHD". Human Mutation. 30 (10): 1449–1459. CiteSeerX 10.1.1.325.8388. doi:10.1002/humu.21091. PMID 19728363. S2CID 14517505.
- ^ Snider, L; Asawachaicharn, A; Tyler, AE; et al. (Jul 1, 2009). "RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy". Human Molecular Genetics. 18 (13): 2414–2430. doi:10.1093/hmg/ddp180. PMC 2694690. PMID 19359275.
- ^ a b Kolata, Gina (19 August 2010). "Reanimated 'Junk' DNA Is Found to Cause Disease". The New York Times. Retrieved 29 August 2010.
- ^ Scionti, I; Greco, F; Ricci, G; et al. (Apr 6, 2012). "Large-scale population analysis challenges the current criteria for the molecular diagnosis of fascioscapulohumeral muscular dystrophy". The American Journal of Human Genetics. 90 (4): 628–635. doi:10.1016/j.ajhg.2012.02.019. PMC 3322229. PMID 22482803.
- ^ Geng, LN; Yao, Z; Snider, L; et al. (Jan 17, 2012). "DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy". Developmental Cell. 22 (1): 38–51. doi:10.1016/j.devcel.2011.11.013. PMC 3264808. PMID 22209328.
- ^ Jones, TI; Chen, JC; Rahimov, F; et al. (Oct 15, 2012). "Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis". Human Molecular Genetics. 21 (20): 4419–4430. doi:10.1093/hmg/dds284. PMC 3459465. PMID 22798623.
- ^ Krom, YD; Thijssen, PE; Young, JM; et al. (Apr 2013). "Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD". PLOS Genetics. 9 (4): e1003415. doi:10.1371/journal.pgen.1003415. PMC 3616921. PMID 23593020.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ferreboeuf, M; Mariot, V; Bessieres, B; et al. (Jan 1, 2014). "DUX4 and DUX4 downstream target genes are expressed in fetal FSHD muscles". Human Molecular Genetics. 23 (1): 171–181. doi:10.1093/hmg/ddt409. PMID 23966205.
- ^ a b Cohen, Justin; DeSimone, Alec; Lek, Monkol; Lek, Angela (October 2020). "Therapeutic Approaches in Facioscapulohumeral Muscular Dystrophy". Trends in Molecular Medicine. 27 (2): 123–137. doi:10.1016/j.molmed.2020.09.008. PMC 8048701. PMID 33092966.
- ^ Tawil, R; McDermott, MP; Pandya, S; King, W; Kissel, J; Mendell, JR; Griggs, RC (January 1997). "A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. FSH-DY Group". Neurology. 48 (1): 46–9. doi:10.1212/wnl.48.1.46. PMID 9008492. S2CID 729275.
- ^ Kissel, JT; McDermott, MP; Natarajan, R; Mendell, JR; Pandya, S; King, WM; Griggs, RC; Tawil, R (May 1998). "Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. FSH-DY Group". Neurology. 50 (5): 1402–6. doi:10.1212/wnl.50.5.1402. PMID 9595995. S2CID 24848310.
- ^ Kissel, JT; McDermott, MP; Mendell, JR; King, WM; Pandya, S; Griggs, RC; Tawil, R; FSH-DY, Group. (23 October 2001). "Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy". Neurology. 57 (8): 1434–40. doi:10.1212/wnl.57.8.1434. PMID 11673585. S2CID 28093111.
- ^ van der Kooi, EL; Vogels, OJ; van Asseldonk, RJ; Lindeman, E; Hendriks, JC; Wohlgemuth, M; van der Maarel, SM; Padberg, GW (24 August 2004). "Strength training and albuterol in facioscapulohumeral muscular dystrophy". Neurology. 63 (4): 702–8. doi:10.1212/01.wnl.0000134660.30793.1f. PMID 15326246. S2CID 22778327.
- ^ a b Campbell, AE; Oliva, J; Yates, MP; Zhong, JW; Shadle, SC; Snider, L; Singh, N; Tai, S; Hiramuki, Y; Tawil, R; van der Maarel, SM; Tapscott, SJ; Sverdrup, FM (4 September 2017). "BET bromodomain inhibitors and agonists of the beta-2 adrenergic receptor identified in screens for compounds that inhibit DUX4 expression in FSHD muscle cells". Skeletal Muscle. 7 (1): 16. doi:10.1186/s13395-017-0134-x. PMC 5584331. PMID 28870238.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Elsheikh, BH; Bollman, E; Peruggia, M; King, W; Galloway, G; Kissel, JT (24 April 2007). "Pilot trial of diltiazem in facioscapulohumeral muscular dystrophy". Neurology. 68 (17): 1428–9. doi:10.1212/01.wnl.0000264017.08217.39. PMID 17452589. S2CID 361422.
- ^ Wyeth Initiates Clinical Trial with Investigational Muscular Dystrophy Therapy MYO-029
- ^ Malcolm, Emily (18 June 2019). "ACE-083". Muscular Dystrophy News. Retrieved 19 December 2019.
- ^ Kinoshita, June. "What's in a name? – FSHD Society". FSHD Society. Retrieved 18 August 2019.
- ^ "FSH Society".
- ^ "FSHD Europe : 光の中のたこ焼きを食べたいと思って30年 – なんのこっちゃ!".
- ^ Rickard, Amanda; Petek, Lisa; Miller, Daniel (August 5, 2015). "Endogenous DUX4 expression in FSHD myotubes is sufficient to cause cell death and disrupts RNA splicing and cell migration pathways". Hum. Mol. Genet. 24 (20): 5901–14. doi:10.1093/hmg/ddv315. PMC 4581613. PMID 26246499.
- ^ "Fulcrum Therapeutics Acquires Global Rights to Losmapimod, a Potential Disease-Modifying Therapy for Facioscapulohumeral Muscular Dystrophy". BioSpace. Retrieved 12 August 2019.
- ^ "Efficacy and Safety of Losmapimod in Subjects With Facioscapulohumeral Muscular Dystrophy (FSHD)". ClinicalTrials.gov. United States National Library of Medicine. Retrieved 12 August 2019.
- ^ "Facio to present at the World Muscle Society Congress". Facio Therapies. 30 September 2019. Retrieved 9 November 2019.
- ^ "Facio reveals novel mechanism targeting the cause of FSHD". Facio Therapies. 24 June 2019. Retrieved 9 November 2019.
- ^ "Arrowhead Pharmaceuticals announces FSHD drug candidate". FSHD Society. 15 April 2021. Retrieved 15 April 2021.
- ^ "Arrowhead Announces ARO-DUX4 as First Muscle Targeted RNAi Candidate Using TRiMTM Platform". Businesswire. 15 April 2021. Retrieved 15 April 2021.
- ^ Joubert, R; Mariot, V; Charpentier, M; Concordet, JP; Dumonceaux, J (23 December 2020). "Gene Editing Targeting the DUX4 Polyadenylation Signal: A Therapy for FSHD?". Journal of Personalized Medicine. 11 (1): 7. doi:10.3390/jpm11010007. PMC 7822190. PMID 33374516.
- ^ DeSimone, Alec M.; Leszyk, John; Wagner, Kathryn; Emerson, Charles P. (11 December 2019). "Identification of the hyaluronic acid pathway as a therapeutic target for facioscapulohumeral muscular dystrophy". Science Advances. 5 (12): eaaw7099. Bibcode:2019SciA....5.7099D. doi:10.1126/sciadv.aaw7099. PMC 6905861. PMID 31844661.
- ^ Bosnakovski, D; da Silva, MT; Sunny, ST; Ener, ET; Toso, EA; Yuan, C; Cui, Z; Walters, MA; Jadhav, A; Kyba, M (September 2019). "A novel P300 inhibitor reverses DUX4-mediated global histone H3 hyperacetylation, target gene expression, and cell death". Science Advances. 5 (9): eaaw7781. Bibcode:2019SciA....5.7781B. doi:10.1126/sciadv.aaw7781. PMC 6739093. PMID 31535023.
- ^ Passerieux, E; Hayot, M; Jaussent, A; Carnac, G; Gouzi, F; Pillard, F; Picot, MC; Böcker, K; Hugon, G; Pincemail, J; Defraigne, JO; Verrips, T; Mercier, J; Laoudj-Chenivesse, D (April 2015). "Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: a double-blind randomized controlled clinical trial" (PDF). Free Radical Biology & Medicine. 81: 158–69. doi:10.1016/j.freeradbiomed.2014.09.014. PMID 25246239.
- ^ Dany, Antoine; Barbe, Coralie; Rapin, Amandine; Réveillère, Christian; Hardouin, Jean-Benoit; Morrone, Isabella; Wolak-Thierry, Aurore; Dramé, Moustapha; Calmus, Arnaud; Sacconi, Sabrina; Bassez, Guillaume; Tiffreau, Vincent; Richard, Isabelle; Gallais, Benjamin; Prigent, Hélène; Taiar, Redha; Jolly, Damien; Novella, Jean-Luc; Boyer, François Constant (2015). "Construction of a Quality of Life Questionnaire for slowly progressive neuromuscular disease". Quality of Life Research. 24 (11): 2615–2623. doi:10.1007/s11136-015-1013-8. ISSN 0962-9343. PMID 26141500. S2CID 25834947.
- ^ Mul, K; Hamadeh, T; Horlings, CGC; Tawil, R; Statland, JM; Sacconi, S; Corbett, AJ; Voermans, NC; Faber, CG; van Engelen, BGM; Merkies, ISJ (10 April 2021). "The FacioScapuloHumeral muscular Dystrophy Rasch-built Overall Disability Scale (FSHD-RODS)". European Journal of Neurology. doi:10.1111/ene.14863. PMID 33838063.
External links
- list of clinical trials for FSHD, Clinicaltrials.gov.
- fsh at NIH/UW GeneTests