Blastomycosis: Difference between revisions
m Fixed CS1 maint: extra punctuation and general fixes |
→History: ++ |
||
| Line 247: | Line 247: | ||
== History == |
== History == |
||
| ⚫ | Blastomycosis was first described by [[Thomas Casper Gilchrist]] in 1894 |
||
The organisms causing blastomycosis have existed for millions of years. The pathogenic group of [[onygenalean]] fungi that give rise to conditions including blastomycosis and histoplasmosis emerged approximately 150 million years ago.<ref name="caballerovandyke-2019">{{Cite journal |
|||
| ⚫ | |||
| journal = Current Opinion in Microbiology |
|||
| volume = 52 |
|||
| date = December 2019 |
|||
| pages = 55-63 |
|||
| author1-first = Marley C |
|||
| author1-last = Caballero Van Dyke |
|||
| author2-first = Marcus M |
|||
| author2-last = Teixeira |
|||
| author3-first = Bridget M |
|||
| author3-last = Barker |
|||
| url = https://www.sciencedirect.com/science/article/abs/pii/S136952741930013X |
|||
| title = Fantastic yeasts and where to find them: the hidden diversity of dimorphic fungal pathogens |
|||
| doi = 10.1016/j.mib.2019.05.002 |
|||
}}</ref> The blastomycosis-causing fungi ''Blastomyces dermatitidis'' and ''Blastomyces gilchristii'' diverged during the [[Pleistocene]], approximately 1.9 million years ago.<ref name="klein2021">{{Cite book |
|||
| title = Encyclopedia of Mycology |
|||
| volume = 1 |
|||
| year = 2021 |
|||
| pages = 638-653 |
|||
| chapter = Blastomyces and Blastomycosis |
|||
| author1-first = Bruce S. |
|||
| author1-last = Klein |
|||
| author2-first = Joseph A. |
|||
| author2-last = McBride |
|||
| author3-first = Gregory M. |
|||
| author3-last = Gauthier |
|||
| doi = 10.1016/B978-0-12-809633-8.21010-8 |
|||
}}</ref> |
|||
| ⚫ | At the [[Koster Site]] in Illinois, evidence pointing to possible blastomycosis infections among [[Woodland period|Late Woodland]] [[Native Americans in the United States|Native Americans]] has been identified. At that site, [[Jane E. Buikstra|Dr. Jane Buikstra]] found evidence for what may have been an epidemic of a serious spinal disease in adolescents and young adults. Several of the skeletons showed lesions in the spinal vertebrae in the lower back. There are two modern diseases that produce lesions in the bone similar to the ones Dr. Buikstra found in these prehistoric specimens - [[Pott disease|spinal TB]] and blastomycosis. The bony lesions in these two diseases are practically identical. Blastomycosis seems more probable as these young people in Late Woodland and Mississippian times may have been afflicted because they were spending more time cultivating plants than their Middle Woodland predecessors had done. If true, it would be another severe penalty Late Woodland people had to pay as they shifted to agriculture as a way of life, and it would be a contributing factor to shortening their lifespans comparedt to those of the Middle Woodland people.<ref>Struever, Stuart and Felicia Antonelli Holton (1979). ''Koster: Americans in Search of Their Prehistoric Past''. New York: Anchor Press / Doubleday. {{ISBN|0-385-00406-0}}.</ref> |
||
| ⚫ | Blastomycosis was first described by [[Thomas Casper Gilchrist]] in 1894, as a skin disease. Because of this, blastomycosis is sometimes called "Gilchrist's disease".<ref name=Gilchrist/> Gilchrist initially identified the cause of the disease as a [[protozoan]], but later identified it as a fungus.<ref name="espinelingroff1996">{{Cite book |
||
| url = https://link.springer.com/book/10.1007/978-94-017-0311-6 |
|||
| doi = 10.1007/978-94-017-0311-6 |
|||
| author-first = Ana Victoria |
|||
| author-last = Espinel-Ingroff |
|||
| title = Medical Mycology in the United States: A Historical Analysis (1894–1996) |
|||
| year = 2003 |
|||
| isbn = 978-94-017-0311-6}}</ref> In 1898 he and [[William Royal Stokes]] published the first description of ''Blastomyces dermatitidis''.<ref name="espinelingroff1996"/> Gilchrist referred to the disease as "blastomycetic dermatitis". |
|||
The systemic spread of blastomycosis was first described in 1902, in a case that had been misdiagnosed as a combination of tuberculosis and a blastomycosis skin infection. The [[dimorphic fungus|dimorphic]] nature of the ''Blastomyces'' fungus was first identified in 1907.<ref name="espinelingroff1996"/> The first case of canine blastomycosis was described in 1912.<ref name="schwartz2018"/> Prior to the 1930s, blastomycosis was not clearly distinguished from similar fungal conditions; a paper by [[Rhoda Williams Benham]] in 1934 distinguished the causative agent of blastomycosis from [[cryptococcosis]] and [[coccidioidomycosis]].<ref name="espinelingroff1996"/> |
|||
In the 1950s, blastomycosis was first determined to be a primarily respiratory disease, with most skin lesions caused by systemic spread from lung infections.<ref>{{Cite book |
|||
| url = https://link.springer.com/chapter/10.1007/978-1-4757-9313-0_3 |
|||
| doi = 10.1007/978-1-4757-9313-0_3 |
|||
| title = Atlas of Infectious Diseases |
|||
| pages = 39–51 |
|||
| chapter = Blastomycosis |
|||
| author-first = Peter G. |
|||
| author-last = Pappas |
|||
}}</ref> In 1952, the first documented case outside North or Central America, in [[Tunisia]], was reported.<ref>{{Cite book |
|||
| url = https://link.springer.com/chapter/10.1007/978-1-59259-296-8_29 |
|||
| doi = 10.1007/978-1-59259-296-8_29 |
|||
| title = Opportunistic Infections |
|||
| pages = 413–427 |
|||
| chapter = Blastomyces dermatitidis |
|||
| author-first = Vassil |
|||
| author-last = St. Georgiev |
|||
}}</ref> The 1950s saw the first introduction of [[antifungal]] drugs including [[amphotericin B]]; prior to 1950, the fatality rate for disseminated blastomycosis was 92%, and treatment options were limited to iodide compounds, radiation therapy, and surgery.<ref name="espinelingroff1996"/> The first azole antifungal drug, [[ketoconazole]], was developed in the 1970s and approved in the United States in 1981.<ref name="espinelingroff1996"/> |
|||
Prior to 2013, the only species known to cause blastomycosis was ''B. dermatitidis''. Since that time, genomic analysis has identified multiple other ''Blastomyces'' species causing blastomycosis, including ''B. gilchristii'' (2013), ''B. helicus'' (reassigned from the genus ''[[Emmonsia]]'' in 2017), ''B. percursus'' (2017), and ''B. emzantsi'' (2020).<ref name="klein2021"/> |
|||
== Other animals == |
== Other animals == |
||
Revision as of 02:38, 19 May 2022
| Blastomycosis | |
|---|---|
| Other names | North American blastomycosis,[1] Chicago disease, Gilchrist's disease[2] |
 | |
| Skin lesions of blastomycosis. | |
| Specialty | Infectious disease[3] |
| Treatment | Antifungals[4] |
| Medication | Itraconazole, amphotericin B[4] |
Blastomycosis is a fungal infection caused by inhaling spores of a Blastomyces fungus.[5] Only about half of people with the disease have symptoms, which can include fever, cough, night sweats, muscle pains, weight loss, chest pain, and feeling tired.[6] These symptoms usually develop between three weeks and three months after breathing in the spores.[6] Although blastomycosis is especially dangerous for those with weak immune systems, most people who contract blastomycosis have healthy immune systems.[7] Most blastomycosis infections affect the lungs.[7] However, in 25 to 40% of cases, the infection also spreads to other parts of the body, such as the skin, bones or central nervous system.[7]
Blastomyces dermatitidis is found in the soil and decaying organic matter like wood or leaves.[4] Participating in outdoor activities like hunting or camping in wooded areas increases the risk of developing blastomycosis.[8] There is no vaccine, but the risk of the disease can be reduced by not disturbing the soil.[8] Treatment is typically with itraconazole for mild or moderate disease; in severe cases, patients are treated with amphotericin B before being placed on itraconazole.[9] With both, the duration of treatment is 6–12 months.[10] Overall, 4-6% of people who develop blastomycosis die; however, if the central nervous system is involved, this rises to 18%. People with AIDS or on medications that suppress the immune system have the highest risk of death at 25-40%.[11]
Blastomycosis is endemic to the eastern United States, especially the Ohio and Mississippi River valleys, the Great Lakes, and the St. Lawrence River. It is also endemic to some parts of Canada, including Quebec, Ontario, and Manitoba.[4] In these areas, there are about 1 to 2 cases per 100,000 per year.[12] It also occurs less frequently in western North America and in Africa, the Middle East, and India.[7][13] Blastomycosis was first described by Thomas Casper Gilchrist in 1894; because of this, it is sometimes called "Gilchrist's disease".[14]
Signs and symptoms
The symptoms of blastomycosis cover a wide range, overlapping with more common conditions; for this reason, blastomycosis has often been called "the great pretender".[7] Many cases are asymptomatic or subclinical. Lung symptoms are common, because the lungs are infected in 79% of blastomycosis cases.[7] However, in 25-40% of cases the disease also disseminates to other organs, including the skin.[7]
The extent and severity of symptoms depends in part on a person's immune status; less than 50% of healthy people with blastomycosis have symptoms, while immunocompromised patients are especially likely to have the disease spread beyond the lungs to other organs like the skin and bones.[15]
Ways in which blastomycosis may present include the following:
- a flu-like illness with fever, chills, arthralgia (joint pain), myalgia (muscle pain), headache, and a nonproductive cough which resolves within days.
- an acute illness resembling bacterial pneumonia, with symptoms of high fever, chills, a productive cough, and pleuritic chest pain.
- a chronic illness that mimics tuberculosis or lung cancer, with symptoms of low-grade fever, a productive cough, night sweats, and weight loss.
- a fast, progressive, and severe disease that manifests as acute respiratory distress syndrome (ARDS), with fever, shortness of breath, tachypnea, hypoxemia, and diffuse pulmonary infiltrates.
- skin lesions, usually asymptomatic, can be verrucous (wart-like) or ulcerated with small pustules at the margins.
- bone lytic lesions can cause bone or joint pain.
- prostatitis may be asymptomatic or may cause pain on urinating.
- laryngeal involvement causes hoarseness.
- 40% of immunocompromised individuals have CNS involvement and present as brain abscess, epidural abscess or meningitis.
Blastomycosis manifests as a primary lung infection in about 79% of cases.[7] The onset is relatively slow and symptoms are suggestive of pneumonia, often leading to initial treatment with antibacterials. Occasionally, if a lesion is seen on X-ray in a cigarette smoker, the disease may be misdiagnosed as carcinoma, leading to swift excision of the pulmonary lobe involved. Upper lung lobes are involved somewhat more frequently than lower lobes.[16] If untreated, many cases progress over a period of months to years to become disseminated blastomycosis. In these cases, the large Blastomyces yeast cells translocate from the lungs and are trapped in capillary beds elsewhere in the body, where they cause lesions. The skin is the most common organ affected, being the site of lesions in approximately 60% of cases.[16] The signature image of blastomycosis in textbooks is the indolent, verrucous or ulcerated dermal lesion seen in disseminated disease. Osteomyelitis is also common (12–60% of cases). Other recurring sites of dissemination are the genitourinary tract (kidney, prostate, epididymis; collectively ca. 25% of cases) and the brain (3–10% of cases).[16]
ARDS is uncommon but very dangerous. For example, this was seen in 9 of 72 blastomycosis cases studied in northeast Tennessee.[17] Such cases may follow massive exposure, such during brush clearing operations. In the Tennessee study, the fatality rate was 89% in the ARDS cases, but only 10% in the non-ARDS cases.[17]
Cause
Blastomycosis is caused by dimorphic fungi in the genus Blastomyces, in the phylum Ascomycota and family Ajellomycetaceae. In eastern North America, the most common cause of blastomycosis is Blastomyces dermatitidis, but Blastomyces gilchristii has been associated with some outbreaks. In western North America, many cases of blastomycosis are caused by Blastomyces helicus, which most commonly attacks immunodeficient people and domestic animals. The species Blastomyces percursus causes many cases of blastomycosis in Africa and the Middle East.[13] In Africa, blastomycosis may also be caused by Blastomyces emzantsi, which is often associated with infections outside the lungs.[18]
In endemic areas, Blastomyces dermatitidis lives in soil and rotten wood near lakes and rivers. Although it has never been directly observed growing in nature, it is thought to grow there as a cottony white mold, similar to the growth seen in artificial culture at 25 °C. The moist, acidic soil in the surrounding woodland harbors the fungus.
Pathogenesis

Inhaled conidia of B. dermatitidis are phagocytosed by neutrophils and macrophages in alveoli. Some of these escape phagocytosis and transform into yeast phase rapidly. Having thick walls, these are resistant to phagocytosis and express glycoprotein, BAD-1, which is a virulence factor as well as an epitope. In lung tissue, they multiply and may disseminate through blood and lymphatics to other organs, including the skin, bone, genitourinary tract, and brain. The incubation period is 30 to 100 days, although infection can be asymptomatic.[citation needed]
Diagnosis
Because the symptoms of blastomycosis resemble those of many other conditions, including tuberculosis and lung cancer, diagnosis is often delayed. In 40% of cases, the diagnosis takes more than a month.[19] A rapid diagnosis can however be made based on microscopic examination of sputum samples or samples obtained from a tissue biopsy or bronchoalveolar lavage.[20]
Once suspected, the diagnosis of blastomycosis can usually be confirmed by demonstration of the characteristic broad based budding organisms in sputum or tissues by KOH prep, cytology, or histology.[21] Tissue biopsy of skin or other organs may be required in order to diagnose extra-pulmonary disease. Blastomycosis is histologically associated with granulomatous nodules.
Commercially available urine antigen testing appears to be quite sensitive in suggesting the diagnosis in cases where the organism is not readily detected.[20] However, commercial antigen tests have a high degree of cross-reactivity with other endemic fungal conditions such as histoplasmosis, and thus cannot distinguish blastomycosis from other similar conditions.[20][22] This cross-reactivity is caused by these related fungal organisms using similar galactomannans in the cell wall.[22]
While culture of the Blastomyces organism remains the definitive diagnostic standard, its slow growing nature can lead to a delay of up to four weeks.[19] In addition, sometimes blood and sputum cultures may not detect blastomycosis.[23] Cultures of the cerebrospinal fluid also have poor sensitivity compared to histopathological examination of the affected tissue.[24]
Treatment
Under Infectious Disease Society of America guidelines, severe cases of blastomycosis and cases with central nervous system (CNS) involvement are treated initially with amphotericin B, followed by a lengthy course of an azole drug such as itraconazole.[9] In most cases the amphotericin treatment lasts for 1–2 weeks, but in cases of CNS involvement it may last for up to 6 weeks.[9] Cases that do not require amphotericin B treatment are treated with a lengthy course of an azole drug.[9]
Among azole drugs, itraconazole is generally the treatment of choice. Voriconazole is often recommended for CNS blastomycosis cases due to its ability to pass the blood-brain barrier.[9] Other azole drugs that may be used include fluconazole. Ketoconazole was the azole drug first used for blastomycosis treatment, but has been largely replaced by itraconazole because ketoconazole is less effective and less tolerated by patients.[9] The azole treatment generally lasts for a minimum of six months. Cure rates from itraconazole treatment are nearly 95%.[9] Relapse is rare but does occur even after a full course of treatment.[9]
Prognosis
Mortality rate in treated cases
- 0-2% in treated cases among immunocompetent patients
- 29% in immunocompromised patients
- 40% in the subgroup of patients with AIDS
- 68% in patients presenting as acute respiratory distress syndrome (ARDS)
Epidemiology

Incidences in most endemic areas are circa 0.5 per 100,000 population, with occasional local areas attaining as high as 12 per 100,000.[16][32][33][34] Most Canadian data fit this picture. In Ontario, Canada, considering both endemic and non-endemic areas, the overall incidence is around 0.3 cases per 100,000; northern Ontario, mostly endemic, has 2.44 per 100,000.[29] Manitoba is calculated at 0.62 cases per 100,000.[25] Remarkably higher incidences were shown for the Kenora, Ontario region: 117 per 100,000 overall, with aboriginal reserve communities experiencing 404.9 per 100,000.[26] In the United States, the incidence of blastomycosis is similarly high in hyperendemic areas. For example, the city of Eagle River, Vilas County, Wisconsin, which has an incidence rate of 101.3 per 100,000; the county as a whole has been shown in two successive studies to have an incidence of ca. 40 cases per 100,000.[35] An incidence of 277 per 100,000 was roughly calculated based on 9 cases seen in a Wisconsin aboriginal reservation during a time in which extensive excavation was done for new housing construction.[36] The new case rates are greater in northern states such as Wisconsin, where from 1986 to 1995 there were 1.4 cases per 100,000 people.[37]
The study of outbreaks as well as trends in individual cases of blastomycosis has clarified a number of important matters. Some of these relate to the ongoing effort to understand the source of infectious inoculum of this species, while others relate to which groups of people are especially likely to become infected. Human blastomycosis is primarily associated with forested areas and open watersheds;[16][38][39][40] It primarily affects otherwise healthy, vigorous people, mostly middle-aged,[41] who acquire the disease while working or undertaking recreational activities in sites conventionally considered clean, healthy and in many cases beautiful.[16][32] Repeatedly associated activities include hunting, especially raccoon hunting,[42] where accompanying dogs also tend to be affected, as well as working with wood or plant material in forested or riparian areas,[16][43] involvement in forestry in highly endemic areas,[44] excavation,[35] fishing[41][45] and possibly gardening and trapping.[26][35]
Urban infections
There is also a developing profile of urban and other domestic blastomycosis cases, beginning with an outbreak tentatively attributed to construction dust in Westmont, Illinois.[46] The city of Rockford, Illinois, was also documented as a hyperendemic area based on incidence rates as high as 6.67 per 100,000 population for some areas of the city. Though proximity to open watersheds was linked to incidence in some areas,[40] suggesting that outdoor activity within the city may be connected to many cases, there is also an increasing body of evidence that even the interiors of buildings may be risk areas. An early case concerned a prisoner who was confined to prison during the whole of his likely blastomycotic incubation period.[47] An epidemiological survey found that although many patients who contracted blastomycosis had engaged in fishing, hunting, gardening, outdoor work and excavation, the most strongly linked association in patients was living or visiting near waterways.[45] Based on a similar finding in a Louisiana study, it has been suggested that place of residence might be the most important single factor in blastomycosis epidemiology in north central Wisconsin.[48] Follow-up epidemiological and case studies indicated that clusters of cases were often associated with particular domiciles, often spread out over a period of years, and that there were uncommon but regularly occurring cases in which pets kept mostly or entirely indoors, in particular cats, contracted blastomycosis.[49][50] The occurrence of blastomycosis, then, is an issue strongly linked to housing and domestic circumstances.
Seasonal trends
Seasonality and weather also appear to be linked to contraction of blastomycosis. Many studies have suggested an association between blastomycosis contraction and cool to moderately warm, moist periods of the spring and autumn[16][26][51] or, in relatively warm winter areas.[52] However, the entire summer or a known summer exposure date is included in the association in some studies.[41][53] Occasional studies fail to detect a seasonal link.[54] In terms of weather, both unusually dry weather[55] and unusually moist weather[56] have been cited. The seemingly contradictory data can most likely be reconciled by proposing that B. dermatitidis prospers in its natural habitats in times of moisture and moderate warmth, but that inoculum formed during these periods remains alive for some time and can be released into the air by subsequent dust formation under dry conditions. Indeed, dust per se or construction potentially linked to dust has been associated with several outbreaks[17][46][57] The data, then, tend to link blastomycosis to all weather, climate and atmospheric conditions except freezing weather, periods of snow cover, and extended periods of hot, dry summer weather in which soil is not agitated.
Gender bias
Sex is another factor inconstantly linked to contraction of blastomycosis: though many studies show more men than women affected,[16][29] some show no sex-related bias.[26][45] As mentioned above, most cases are in middle aged adults, but all age groups are affected, and cases in children are not uncommon.[16][26][29]
Ethnic populations
Ethnic group or race is frequently investigated in epidemiological studies of blastomycosis, but is potentially confounded by differences in residence and in quality and accessibility of medical care, factors that have not been stringently controlled for to date. In the United States, some studies show a disproportionately high incidence and/or mortality rate for blastomycosis among Black people.[33][34][58][59]
In Canada, some studies, but not others,[25] indicate that First Nations people have a disproportionately high incidence of blastomycosis.[26][60] Incidence in First Nations children may be unusually high.[26] The Canadian data in some areas may be confounded or explained by the tendency to establish indigenous communities in wooded, riparian, northern areas corresponding to the core habitat of B. dermatitidis, often with known B. dermatitidis habitats such as woodpiles and beaver constructions in the near vicinity.
Communicability
Blastomycosis is not considered contagious, either among humans or between animals and humans.[8] However, there are a very small number of cases of human-to-human transmission of B. dermatitidis related to dermal contact[61] or sexual transmission of disseminated blastomycosis of the genital tract among spouses.[16]
History
The organisms causing blastomycosis have existed for millions of years. The pathogenic group of onygenalean fungi that give rise to conditions including blastomycosis and histoplasmosis emerged approximately 150 million years ago.[62] The blastomycosis-causing fungi Blastomyces dermatitidis and Blastomyces gilchristii diverged during the Pleistocene, approximately 1.9 million years ago.[63]
At the Koster Site in Illinois, evidence pointing to possible blastomycosis infections among Late Woodland Native Americans has been identified. At that site, Dr. Jane Buikstra found evidence for what may have been an epidemic of a serious spinal disease in adolescents and young adults. Several of the skeletons showed lesions in the spinal vertebrae in the lower back. There are two modern diseases that produce lesions in the bone similar to the ones Dr. Buikstra found in these prehistoric specimens - spinal TB and blastomycosis. The bony lesions in these two diseases are practically identical. Blastomycosis seems more probable as these young people in Late Woodland and Mississippian times may have been afflicted because they were spending more time cultivating plants than their Middle Woodland predecessors had done. If true, it would be another severe penalty Late Woodland people had to pay as they shifted to agriculture as a way of life, and it would be a contributing factor to shortening their lifespans comparedt to those of the Middle Woodland people.[64]
Blastomycosis was first described by Thomas Casper Gilchrist in 1894, as a skin disease. Because of this, blastomycosis is sometimes called "Gilchrist's disease".[14] Gilchrist initially identified the cause of the disease as a protozoan, but later identified it as a fungus.[65] In 1898 he and William Royal Stokes published the first description of Blastomyces dermatitidis.[65] Gilchrist referred to the disease as "blastomycetic dermatitis".
The systemic spread of blastomycosis was first described in 1902, in a case that had been misdiagnosed as a combination of tuberculosis and a blastomycosis skin infection. The dimorphic nature of the Blastomyces fungus was first identified in 1907.[65] The first case of canine blastomycosis was described in 1912.[66] Prior to the 1930s, blastomycosis was not clearly distinguished from similar fungal conditions; a paper by Rhoda Williams Benham in 1934 distinguished the causative agent of blastomycosis from cryptococcosis and coccidioidomycosis.[65]
In the 1950s, blastomycosis was first determined to be a primarily respiratory disease, with most skin lesions caused by systemic spread from lung infections.[67] In 1952, the first documented case outside North or Central America, in Tunisia, was reported.[68] The 1950s saw the first introduction of antifungal drugs including amphotericin B; prior to 1950, the fatality rate for disseminated blastomycosis was 92%, and treatment options were limited to iodide compounds, radiation therapy, and surgery.[65] The first azole antifungal drug, ketoconazole, was developed in the 1970s and approved in the United States in 1981.[65]
Prior to 2013, the only species known to cause blastomycosis was B. dermatitidis. Since that time, genomic analysis has identified multiple other Blastomyces species causing blastomycosis, including B. gilchristii (2013), B. helicus (reassigned from the genus Emmonsia in 2017), B. percursus (2017), and B. emzantsi (2020).[63]
Other animals
Blastomycosis affects a broad range of mammals. As with humans, most animals that become infectecd were formerly healthy and immunocompetent.[66] Dogs are especially common victims; blastomycosis is eight to ten times more common in dogs than in humans.[66] Cats and horses can also be infected, and cats with feline immunodeficiency virus are particularly at risk.[66] Cases of blastomycosis have been reported from captive lions and tigers, in a wild North American black bear, and in marine mammals such as the Atlantic bottlenose dolphin.[66]
The nonspecific symptoms that make blastomycosis difficult to diagnose in humans also complicate veterinary diagnosis. Cats in particular are often only diagnosed after death.[66]
Dogs and humans frequently become infected from the same exposure.[66] In most such cases, the infection in the dog becomes apparent before the human infection.[66] This may be due to a shortened incubation period, due to the dog inhaling larger quantities of Blastomyces spores than the human.[66]
In veterinary care, blastomycosis is typically treated with itraconazole.[69] 70% of infected dogs treated with itraconazole respond to medication and recover.[69] In dogs as in humans, the prognosis for blastomycosis depends on the severity of the symptoms.[69]
Additional images
-
Granuloma with early suppuration. Fungal organisms difficult to recognize at this low magnification.
-
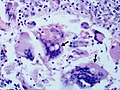
Large yeast-like fungi seen within giant cells at arrows.
-
Large yeast-like fungi seen within giant cells at arrows.Budding yeasts in cytoplasm of giant cells at arrows. Broad-based budding and double contoured cell wall seen in the giant cell in the center is characteristic of Blastomyces dermatitidis.
-
Nodular skin lesions of blastomycosis, one of which is a bullous lesion on top of a nodule.
See also
References
- ^ Johnstone, Ronald B. (2017). "25. Mycoses and Algal infections". Weedon's Skin Pathology Essentials (2nd ed.). Elsevier. p. 449. ISBN 978-0-7020-6830-0.
- ^ Calderone, Richard (2002). Fungal Pathogenesis: Principles and Clinical Applications. Boca Raton: CRC Press. doi:10.1201/9781482270907. ISBN 9780429153228.
- ^ "ICD-11 - ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Retrieved 29 May 2021.
- ^ a b c d "Information for Healthcare Professionals about Blastomycosis". cdc.gov. 2019-01-24. Archived from the original on 26 April 2019. Retrieved 15 May 2019.
- ^ "Blastomycosis". cdc.gov. Centers for Disease Control and Prevention. Retrieved 2022-05-14.
- ^ a b "Symptoms of Blastomycosis". cdc.gov. 2019-01-29. Archived from the original on 26 April 2019. Retrieved 15 May 2019.
- ^ a b c d e f g h McBride, Joseph A.; Gauthier, Gregory M.; Klein, Bruce S. (September 2017). "Clinical manifestations and treatment of blastomycosis". Clinical Chest Medicine. 38 (3): 435–449. doi:10.1016/j.ccm.2017.04.006. PMC 5657236. PMID 28797487.
- ^ a b c "Blastomycosis Risk & Prevention". cdc.gov. 2019-01-29. Archived from the original on 26 April 2019. Retrieved 22 May 2019.
- ^ a b c d e f g h Chapman, Stanley W.; Dismukes, William E.; Proia, Laurie A.; Bradsher, Robert W.; Pappas, Peter G.; Threlkeld, Michael G.; Kauffman, Carol A. (2008-06-15). "Clinical Practice Guidelines for the Management of Blastomycosis: 2008 Update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 46 (12): 1801–1812. doi:10.1086/588300.
- ^ "Treatment for Blastomycosis". cdc.gov. 2019-01-29. Archived from the original on 26 April 2019. Retrieved 22 May 2019.
- ^ Castillo CG, Kauffman CA, Miceli MH (March 2016). "Blastomycosis". Infectious Disease Clinics of North America. 30 (1): 247–64. doi:10.1016/j.idc.2015.10.002. PMID 26739607.
- ^ "Blastomycosis Statistics". cdc.gov. 2019-01-24. Archived from the original on 26 April 2019. Retrieved 22 May 2019.
- ^ a b Schwartz, Ilan S; et al. (2021-10-01). "Blastomycosis in Africa and the Middle East: A Comprehensive Review of Reported Cases and Reanalysis of Historical Isolates Based on Molecular Data". Clinical Infectious Diseases. 73 (7): e1560–e1569. doi:10.1093/cid/ciaa1100. PMC 8492124. PMID 32766820. Retrieved 2022-05-14.
- ^ a b Crissey, John Thorne; Parish, Lawrence C.; Holubar, Karl (2002). Historical Atlas of Dermatology and Dermatologists. Parthenon Publishing Group. p. 86. ISBN 978-1842141007.
- ^ Murray P, Rosenthal K, Pfaller M (2015). "Chapter 64: Systemic Mycoses Caused by Dimorphic Fungi". Medical Microbiology (8 ed.). Elsevier. pp. 629–633. ISBN 978-0323299565.
- ^ a b c d e f g h i j k l Kwon-Chung, K.J., Bennett, J.E.; Bennett, John E. (1992). Medical mycology. Philadelphia: Lea & Febiger. ISBN 978-0812114638.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Vasquez, JE; Mehta, JB; Agrawal, R; Sarubbi, FA (1998). "Blastomycosis in northeast Tennessee". Chest. 114 (2): 436–43. doi:10.1378/chest.114.2.436. PMID 9726727.
- ^ Borman, Andrew M.; Johnson, Elizabeth M. (February 2021). "Name Changes for Fungi of Medical Importance, 2018 to 2019". Journal of Clinical Microbiology. 59 (2): e01811–20. doi:10.1128/JCM.01811-20. PMC 8111128. PMID 33028600.
- ^ a b Mazi, Patrick B.; Rauseo, Adriana M.; Spec, Andrej (June 2021). "Blastomycosis". Infectious Disease Clinics of North America. 35 (2): 515–530.
- ^ a b c Nel, J. S.; Bartelt, L. A.; van Duin, D.; Lachiewicz, A. M. (2018). "Endemic Mycoses in Solid Organ Transplant Recipients". Infectious disease clinics of North America. 32 (3): 667–685. doi:10.1016/j.idc.2018.04.007.
- ^ Veligandla SR, Hinrichs SH, Rupp ME, Lien EA, Neff JR, Iwen PC (October 2002). "Delayed diagnosis of osseous blastomycosis in two patients following environmental exposure in nonendemic areas". Am. J. Clin. Pathol. 118 (4): 536–41. doi:10.1309/JEJ0-3N98-C3G8-21DE. PMID 12375640.
- ^ a b Linder, Kathleen A.; Kauffman, Carol A. (2021). "Current and New Perspectives in the Diagnosis of Blastomycosis and Histoplasmosis". Journal of Fungi. 7 (1): 12. doi:10.3390/jof7010012.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Morgan, Matthew W; Salit, Irving E (1996). "Human and canine blastomycosis: A common source infection". The Canadian Journal of Infectious Diseases. 7 (2): 147–151. doi:10.1155/1996/657941. ISSN 1180-2332. PMC 3327387. PMID 22514432.
- ^ Majdick, K.; Kaye, K.; Shorman, M. A. (2020). "Central nervous system blastomycosis clinical characteristics and outcomes". Medical Mycology. doi:10.1093/mmy/myaa041.
- ^ a b c Crampton, TL; Light, RB; Berg, GM; Meyers, MP; Schroeder, GC; Hershfield, ES; Embil, JM (2002). "Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals". Clinical Infectious Diseases. 34 (10): 1310–6. doi:10.1086/340049. PMID 11981725.
- ^ a b c d e f g h Dwight, P.J.; Naus, M; Sarsfield, P; Limerick, B (2000). "An outbreak of human blastomycosis: the epidemiology of blastomycosis in the Kenora catchment region of Ontario, Canada". Canada Communicable Disease Report. 26 (10): 82–91. PMID 10893821.
- ^ Kane, J; Righter, J; Krajden, S; Lester, RS (1983). "Blastomycosis: a new endemic focus in Canada". Canadian Medical Association Journal. 129 (7): 728–31. PMC 1875443. PMID 6616383.
- ^ Lester, RS; DeKoven, JG; Kane, J; Simor, AE; Krajden, S; Summerbell, RC (2000). "Novel cases of blastomycosis acquired in Toronto, Ontario". CMAJ : Canadian Medical Association Journal. 163 (10): 1309–12. PMC 80342. PMID 11107469.
- ^ a b c d Morris, SK; Brophy, J; Richardson, SE; Summerbell, R; Parkin, PC; Jamieson, F; Limerick, B; Wiebe, L; Ford-Jones, EL (2006). "Blastomycosis in Ontario, 1994-2003". Emerging Infectious Diseases. 12 (2): 274–9. doi:10.3201/eid1202.050849. PMC 3373107. PMID 16494754.
- ^ Sekhon, AS; Jackson, FL; Jacobs, HJ (1982). "Blastomycosis: report of the first case from Alberta Canada". Mycopathologia. 79 (2): 65–9. doi:10.1007/bf00468081. PMID 6813742. S2CID 27296444.
- ^ Vallabh, V; Martin, T; Conly, JM (1988). "Blastomycosis in Saskatchewan". The Western Journal of Medicine. 148 (4): 460–2. PMC 1026149. PMID 3388850.
- ^ a b Rippon, John Willard (1988). Medical mycology : the pathogenic fungi and the pathogenic actinomycetes (3rd ed.). Philadelphia: W.B. Saunders Co. ISBN 9780721624440.
- ^ a b Manetti, AC (1991). "Hyperendemic urban blastomycosis". American Journal of Public Health. 81 (5): 633–6. doi:10.2105/ajph.81.5.633. PMC 1405080. PMID 2014867.
- ^ a b Cano, MV; Ponce-de-Leon, GF; Tippen, S; Lindsley, MD; Warwick, M; Hajjeh, RA (2003). "Blastomycosis in Missouri: epidemiology and risk factors for endemic disease". Epidemiology and Infection. 131 (2): 907–14. doi:10.1017/s0950268803008987. PMC 2870035. PMID 14596532.
- ^ a b c Baumgardner, DJ; Brockman, K (1998). "Epidemiology of human blastomycosis in Vilas County, Wisconsin. II: 1991-1996". WMJ. 97 (5): 44–7. PMID 9617309.
- ^ Baumgardner, DJ; Egan, G; Giles, S; Laundre, B (2002). "An outbreak of blastomycosis on a United States Indian reservation". Wilderness & Environmental Medicine. 13 (4): 250–2. doi:10.1580/1080-6032(2002)013[0250:aooboa]2.0.co;2. PMID 12510781.
- ^ Centers for Disease Control and Prevention (CDC) (1996). "Blastomycosis--Wisconsin, 1986-1995". MMWR Morb. Mortal. Wkly. Rep. 45 (28): 601–3. PMID 8676851.
- ^ DiSalvo, A.F. (1992). Al-Doory, Y.; DiSalvo, A.F. (eds.). Ecology of Blastomyces dermatitidis. Plenum. pp. 43–73.
- ^ Baumgardner, DJ; Steber, D; Glazier, R; Paretsky, DP; Egan, G; Baumgardner, AM; Prigge, D (2005). "Geographic information system analysis of blastomycosis in northern Wisconsin, USA: waterways and soil". Medical Mycology. 43 (2): 117–25. doi:10.1080/13693780410001731529. PMID 15832555.
- ^ a b Baumgardner, DJ; Knavel, EM; Steber, D; Swain, GR (2006). "Geographic distribution of human blastomycosis cases in Milwaukee, Wisconsin, USA: association with urban watersheds". Mycopathologia. 161 (5): 275–82. doi:10.1007/s11046-006-0018-9. PMID 16649077. S2CID 7953521.
- ^ a b c Klein, Bruce S.; Vergeront, James M.; Weeks, Robert J.; Kumar, U. Nanda; Mathai, George; Varkey, Basil; Kaufman, Leo; Bradsher, Robert W.; Stoebig, James F.; Davis, Jeffrey P. (1986). "Isolation of Blastomyces dermatitidis in Soil Associated with a Large Outbreak of Blastomycosis in Wisconsin". New England Journal of Medicine. 314 (9): 529–534. doi:10.1056/NEJM198602273140901. PMID 3945290.
- ^ Armstrong, CW; Jenkins, SR; Kaufman, L; Kerkering, TM; Rouse, BS; Miller GB, Jr (1987). "Common-source outbreak of blastomycosis in hunters and their dogs". The Journal of Infectious Diseases. 155 (3): 568–70. doi:10.1093/infdis/155.3.568. PMID 3805778.
- ^ Kesselman, EW; Moore, S; Embil, JM (2005). "Using local epidemiology to make a difficult diagnosis: a case of blastomycosis". CJEM. 7 (3): 171–3. doi:10.1017/S1481803500013221. PMID 17355674.
- ^ Vaaler, AK; Bradsher, RW; Davies, SF (1990). "Evidence of subclinical blastomycosis in forestry workers in northern Minnesota and northern Wisconsin". The American Journal of Medicine. 89 (4): 470–6. doi:10.1016/0002-9343(90)90378-q. PMID 2220880.
- ^ a b c Baumgardner, DJ; Buggy, BP; Mattson, BJ; Burdick, JS; Ludwig, D (1992). "Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin". Clinical Infectious Diseases. 15 (4): 629–35. doi:10.1093/clind/15.4.629. PMID 1420675.
- ^ a b Kitchen, MS; Reiber, CD; Eastin, GB (1977). "An urban epidemic of North American blastomycosis". The American Review of Respiratory Disease. 115 (6): 1063–6. doi:10.1164/arrd.1977.115.6.1063 (inactive 28 February 2022). PMID 262101.
{{cite journal}}: CS1 maint: DOI inactive as of February 2022 (link) - ^ Renston, JP; Morgan, J; DiMarco, AF (1992). "Disseminated miliary blastomycosis leading to acute respiratory failure in an urban setting". Chest. 101 (5): 1463–5. doi:10.1378/chest.101.5.1463. PMID 1582324.
- ^ Lowry, PW; Kelso, KY; McFarland, LM (1989). "Blastomycosis in Washington Parish, Louisiana, 1976-1985". American Journal of Epidemiology. 130 (1): 151–9. doi:10.1093/oxfordjournals.aje.a115307. PMID 2787106.
- ^ Blondin, N; Baumgardner, DJ; Moore, GE; Glickman, LT (2007). "Blastomycosis in indoor cats: suburban Chicago, Illinois, USA". Mycopathologia. 163 (2): 59–66. doi:10.1007/s11046-006-0090-1. PMID 17262169. S2CID 1227756.
- ^ Baumgardner, DJ; Paretsky, DP (2001). "Blastomycosis: more evidence for exposure near one's domicile". WMJ. 100 (7): 43–5. PMID 11816782.
- ^ Rudmann, DG; Coolman, BR; Perez, CM; Glickman, LT (1992). "Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990)". Journal of the American Veterinary Medical Association. 201 (11): 1754–9. PMID 1293122.
- ^ Arceneaux, KA; Taboada, J; Hosgood, G (1998). "Blastomycosis in dogs: 115 cases (1980-1995)". Journal of the American Veterinary Medical Association. 213 (5): 658–64. PMID 9731260.
- ^ Archer, JR; Trainer, DO; Schell, RF (1987). "Epidemiologic study of canine blastomycosis in Wisconsin". Journal of the American Veterinary Medical Association. 190 (10): 1292–5. PMID 3583882.
- ^ Chapman, SW; Lin, AC; Hendricks, KA; Nolan, RL; Currier, MM; Morris, KR; Turner, HR (1997). "Endemic blastomycosis in Mississippi: epidemiological and clinical studies". Seminars in Respiratory Infections. 12 (3): 219–28. PMID 9313293.
- ^ Proctor, ME; Klein, BS; Jones, JM; Davis, JP (2002). "Cluster of pulmonary blastomycosis in a rural community: evidence for multiple high-risk environmental foci following a sustained period of diminished precipitation". Mycopathologia. 153 (3): 113–20. doi:10.1023/A:1014515230994. PMID 11998870. S2CID 38668503.
- ^ De Groote, MA; Bjerke, R; Smith, H; Rhodes III, LV (2000). "Expanding epidemiology of blastomycosis: clinical features and investigation of 2 cases in Colorado". Clinical Infectious Diseases. 30 (3): 582–4. doi:10.1086/313717. PMID 10722448.
- ^ Baumgardner, DJ; Burdick, JS (1991). "An outbreak of human and canine blastomycosis". Reviews of Infectious Diseases. 13 (5): 898–905. doi:10.1093/clinids/13.5.898. PMID 1962106.
- ^ Dworkin, MS; Duckro, AN; Proia, L; Semel, JD; Huhn, G (2005). "The epidemiology of blastomycosis in Illinois and factors associated with death". Clinical Infectious Diseases. 41 (12): e107–11. doi:10.1086/498152. PMID 16288388.
- ^ Lemos, LB; Guo, M; Baliga, M (2000). "Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi". Annals of Diagnostic Pathology. 4 (6): 391–406. doi:10.1053/adpa.2000.20755. PMID 11149972.
- ^ Kepron, MW; Schoemperlen; Hershfield, ES; Zylak, CJ; Cherniack, RM (1972). "North American blastomycosis in Central Canada. A review of 36 cases". Canadian Medical Association Journal. 106 (3): 243–6. PMC 1940364. PMID 5057959.
- ^ Bachir, J; Fitch, GL (2006). "Northern Wisconsin married couple infected with blastomycosis". WMJ. 105 (6): 55–7. PMID 17042422.
- ^ Caballero Van Dyke, Marley C; Teixeira, Marcus M; Barker, Bridget M (December 2019). "Fantastic yeasts and where to find them: the hidden diversity of dimorphic fungal pathogens". Current Opinion in Microbiology. 52: 55–63. doi:10.1016/j.mib.2019.05.002.
- ^ a b Klein, Bruce S.; McBride, Joseph A.; Gauthier, Gregory M. (2021). "Blastomyces and Blastomycosis". Encyclopedia of Mycology. Vol. 1. pp. 638–653. doi:10.1016/B978-0-12-809633-8.21010-8.
- ^ Struever, Stuart and Felicia Antonelli Holton (1979). Koster: Americans in Search of Their Prehistoric Past. New York: Anchor Press / Doubleday. ISBN 0-385-00406-0.
- ^ a b c d e f Espinel-Ingroff, Ana Victoria (2003). Medical Mycology in the United States: A Historical Analysis (1894–1996). doi:10.1007/978-94-017-0311-6. ISBN 978-94-017-0311-6.
- ^ a b c d e f g h i Schwartz, Ilan S. (2018). Seyedmousavi, S.; de Hoog, G.; Guillot, J.; Verweij, P. (eds.). Blastomycosis in Mammals. Springer. doi:10.1007/978-3-319-72093-7_8. ISBN 978-3-319-72093-7.
{{cite book}}:|work=ignored (help) - ^ Pappas, Peter G. "Blastomycosis". Atlas of Infectious Diseases. pp. 39–51. doi:10.1007/978-1-4757-9313-0_3.
- ^ St. Georgiev, Vassil. "Blastomyces dermatitidis". Opportunistic Infections. pp. 413–427. doi:10.1007/978-1-59259-296-8_29.
- ^ a b c "Blastomycosis - Generalized Conditions - Merck Veterinary Manual". Retrieved 2022-05-18.
Further reading
- Castillo CG, Kauffman CA, Miceli MH (2016). "Blastomycosis". Infectious Disease Clinics of North America. 30 (1): 247–264. doi:10.1016/j.idc.2015.10.002. PMID 26739607. (Review).
- McBride JA, Gauthier GM, Klein BS (2017). "Clinical Manifestations and Treatment of Blastomycosis". Clinics in Chest Medicine. 38 (3): 435–449. doi:10.1016/j.ccm.2017.04.006. PMC 5657236. PMID 28797487. PMC 5657236 (Review)
External links
- Blastomyces at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- NIH Encyclopedia Blastomycosis