Carbon sequestration: Difference between revisions
repeated a ref here from the main text. |
→Mineral sequestration: moved from carbon capture and storage, will need proper merging in second step |
||
| Line 143: | Line 143: | ||
=== Mineral carbonation === |
=== Mineral carbonation === |
||
{{update section|date=June 2019}} |
|||
| ⚫ | Carbon, in the form of {{chem|CO|2}} can be removed from the atmosphere by chemical processes, and stored in stable [[carbonate mineral]] forms. This process ({{chem|CO|2}}-to-stone) is known as "carbon sequestration by mineral [[carbonation]]" or mineral sequestration. The process involves reacting carbon dioxide with abundantly available metal oxides – either [[magnesium oxide]] (MgO) or [[calcium oxide]] (CaO) – to form stable carbonates. These reactions are [[exothermic]] and occur naturally (e.g., the [[weathering]] of rock over [[geologic time]] periods).<ref name=herzog/><ref name=goldberg>{{Cite web|title=Conference Proceedings|url=http://www.netl.doe.gov/events/conference-proceedings|access-date=2021-12-30|website=netl.doe.gov|language=en}}</ref> |
||
CO<sub>2</sub> [[Exothermic|exothermically]] reacts with metal oxides, producing stable carbonates (e.g. [[calcite]], [[magnesite]]). This process (CO<sub>2</sub>-to-stone) occurs naturally over periods of years and is responsible for much surface [[limestone]]. [[Olivine]] is one such metal oxide.<ref name="Olivine weathering2">{{cite web |last=Schuiling |first=Olaf |title=Olaf Schuiling proposes olivine rock grinding |url=http://www.gather.com/viewArticle.action?articleId=281474979949059 |url-status=dead |archive-url=https://archive.today/20130411003510/http://www.gather.com/viewArticle.action?articleId=281474979949059 |archive-date=11 April 2013 |access-date=23 December 2011}}</ref>{{self-published inline|date=March 2013}} Rocks rich in metal oxides that react with CO<sub>2</sub>, such as [[Magnesium oxide|MgO]] and [[Calcium oxide|CaO]] as contained in [[Basalt|basalts]], have been proven as a viable means to achieve carbon-dioxide mineral storage.<ref>{{cite journal |last1=Snæbjörnsdóttir |first1=Sandra Ó. |last2=Sigfússon |first2=Bergur |last3=Marieni |first3=Chiara |last4=Goldberg |first4=David |last5=Gislason |first5=Sigurður R. |last6=Oelkers |first6=Eric H. |date=2020 |title=Carbon dioxide storage through mineral carbonation |url=https://hal.archives-ouvertes.fr/hal-03384454/file/Oelkers_%2520Mineral%2520Storage%2520of%2520Carbon%2520Dioxide_AAM-2.pdf |journal=Nature Reviews Earth & Environment |volume=1 |issue=2 |pages=90–102 |bibcode=2020NRvEE...1...90S |doi=10.1038/s43017-019-0011-8 |s2cid=210716072}}</ref><ref>{{cite journal |last1=McGrail |first1=B. Peter |last2=Spane |first2=F.A. |last3=Amonette |first3=J.E. |last4=Thompson |first4=C.R. |display-authors=1 |date=2014 |title=Injection and Monitoring at the Wallula Basalt Pilot Project |journal=Energy Procedia |volume=63 |pages=2939–2948 |doi=10.1016/j.egypro.2014.11.316}}</ref> The reaction rate can in principle be accelerated with a [[catalyst]]<ref>{{cite journal |last1=Bhaduri |first1=Gaurav A. |last2=Šiller |first2=Lidija |date=2013 |title=Nickel nanoparticles catalyse reversible hydration of CO<sub>2</sub> for mineralization carbon capture and storage |journal=Catalysis Science & Technology |volume=3 |issue=5 |pages=1234 |doi=10.1039/C3CY20791A}}</ref> or by increasing temperatures {{dubious|date=December 2022|reason=how can an increase in T increase the rate of an exothermic reaction?}} and/or pressures, or by mineral pre-treatment, although this method can require additional energy. The [[Intergovernmental Panel on Climate Change|IPCC]] estimates that a power plant equipped with CCS using mineral storage would need 60–180% more energy than one without.<ref name="IPCC_CC2">[IPCC, 2005] ''IPCC special report on CO<sub>2</sub> Capture and Storage''. Prepared by working group III of the Intergovernmental Panel on Climate Change. Metz, B., O. Davidson, H. C. de Coninck, M. Loos, and L.A. Meyer (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 442 pp. Available in full at [http://www.ipcc.ch/pdf/special-reports/srccs/srccs_wholereport.pdf www.ipcc.ch] {{Webarchive|url=https://web.archive.org/web/20100210022620/http://www1.ipcc.ch/pdf/special-reports/srccs/srccs_wholereport.pdf|date=10 February 2010}} (PDF - 22.8MB)</ref> Theoretically, up to 22% of crustal mineral mass is able to form [[Carbonate|carbonates]].{{citation needed|date=December 2022}} |
|||
{| class="wikitable" |
|||
|+Selected metal oxides of [[Crust (geology)|Earth's crust]] |
|||
!Earthen oxide |
|||
!Percent of crust |
|||
!Carbonate |
|||
![[Standard enthalpy change of reaction|Enthalpy change]] (kJ/mol) |
|||
|- |
|||
|[[CaO]] |
|||
|4.90 |
|||
|[[Calcium carbonate|CaCO<sub>3</sub>]] |
|||
|−179 |
|||
|- |
|||
|[[MgO]] |
|||
|4.36 |
|||
|[[Magnesium carbonate|MgCO<sub>3</sub>]] |
|||
|−118 |
|||
|- |
|||
|[[Sodium oxide|Na<sub>2</sub>O]] |
|||
|3.55 |
|||
|[[Sodium carbonate|Na<sub>2</sub>CO<sub>3</sub>]] |
|||
|−322 |
|||
|- |
|||
|[[FeO]] |
|||
|3.52 |
|||
|[[Iron carbonate|FeCO<sub>3</sub>]] |
|||
|−85 |
|||
|- |
|||
|[[Potassium oxide|K<sub>2</sub>O]] |
|||
|2.80 |
|||
|[[Potassium carbonate|K<sub>2</sub>CO<sub>3</sub>]] |
|||
|−393.5 |
|||
|- |
|||
|[[Fe2O3|Fe<sub>2</sub>O<sub>3</sub>]] |
|||
|2.63 |
|||
|[[Iron carbonate|FeCO<sub>3</sub>]] |
|||
|112 |
|||
|- |
|||
|All oxides |
|||
|21.76 |
|||
|All carbonates |
|||
|} |
|||
[[Ultramafic rock|Ultramafic]] [[mine tailings]] are a readily available source of fine-grained metal oxides that could serve this purpose.<ref>{{cite journal |last1=Wilson |first1=Siobhan A. |last2=Dipple |first2=Gregory M. |last3=Power |first3=Ian M. |last4=Thom |first4=James M. |last5=Anderson |first5=Robert G. |last6=Raudsepp |first6=Mati |last7=Gabites |first7=Janet E. |last8=Southam |first8=Gordon |year=2009 |title=CO<sub>2</sub> Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada |journal=Economic Geology |volume=104 |pages=95–112 |doi=10.2113/gsecongeo.104.1.95}}</ref> Accelerating passive CO<sub>2</sub> sequestration via mineral carbonation may be achieved through microbial processes that enhance mineral dissolution and carbonate precipitation.<ref>{{cite journal |last1=Power |first1=Ian M. |last2=Dipple |first2=Gregory M. |last3=Southam |first3=Gordon |year=2010 |title=Bioleaching of Ultramafic Tailings by ''Acidithiobacillus'' spp. For CO<sub>2</sub> Sequestration |journal=Environmental Science & Technology |volume=44 |issue=1 |pages=456–62 |bibcode=2010EnST...44..456P |doi=10.1021/es900986n |pmid=19950896}}</ref><ref>{{cite journal |last1=Power |first1=Ian M |last2=Wilson |first2=Siobhan A |last3=Thom |first3=James M |last4=Dipple |first4=Gregory M |last5=Southam |first5=Gordon |year=2007 |title=Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada |journal=Geochemical Transactions |volume=8 |page=13 |doi=10.1186/1467-4866-8-13 |pmc=2213640 |pmid=18053262}}</ref><ref>{{cite journal |last1=Power |first1=Ian M. |last2=Wilson |first2=Siobhan A. |last3=Small |first3=Darcy P. |last4=Dipple |first4=Gregory M. |last5=Wan |first5=Wankei |last6=Southam |first6=Gordon |year=2011 |title=Microbially Mediated Mineral Carbonation: Roles of Phototrophy and Heterotrophy |journal=Environmental Science & Technology |volume=45 |issue=20 |pages=9061–8 |bibcode=2011EnST...45.9061P |doi=10.1021/es201648g |pmid=21879741}}</ref> |
|||
| ⚫ | Carbon, in the form of {{chem|CO|2}} can be removed from the atmosphere by chemical processes, and stored in stable [[carbonate mineral]] forms. This process ({{chem|CO|2}}-to-stone) is known as "carbon sequestration by mineral [[carbonation]]" or mineral sequestration. The process involves reacting carbon dioxide with abundantly available metal oxides – either [[magnesium oxide]] (MgO) or [[calcium oxide]] (CaO) – to form stable carbonates. These reactions are [[exothermic]] and occur naturally (e.g., the [[weathering]] of rock over [[geologic time]] periods).<ref name="herzog" /><ref name="goldberg">{{Cite web|title=Conference Proceedings|url=http://www.netl.doe.gov/events/conference-proceedings|access-date=2021-12-30|website=netl.doe.gov|language=en}}</ref> |
||
:CaO + {{chem|CO|2}} → {{chem|CaCO|3}} |
:CaO + {{chem|CO|2}} → {{chem|CaCO|3}} |
||
Revision as of 12:22, 6 February 2023
| Part of a series on the |
| Carbon cycle |
|---|
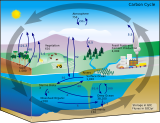 |
Carbon sequestration is the process of storing carbon in a carbon pool.[2]: 2248 Carbon dioxide (CO
2) is naturally captured from the atmosphere through biological, chemical, and physical processes.[3] These changes can be accelerated through changes in land use and agricultural practices, such as converting crop land into land for non-crop fast growing plants.[4] Artificial processes have been devised to produce similar effects,[3] including large-scale, artificial capture and sequestration of industrially produced CO
2 using subsurface saline aquifers, reservoirs, ocean water, aging oil fields, or other carbon sinks, bio-energy with carbon capture and storage, biochar, enhanced weathering, direct air capture and water capture when combined with storage.[5]
Forests, kelp beds, and other forms of plant life absorb carbon dioxide from the air as they grow, and bind it into biomass. However, these biological stores are considered volatile carbon sinks as the long-term sequestration cannot be guaranteed. For example, natural events, such as wildfires or disease, economic pressures and changing political priorities can result in the sequestered carbon being released back into the atmosphere.[6] Carbon dioxide that has been removed from the atmosphere can also be stored in the Earth's crust by injecting it into the subsurface, or in the form of insoluble carbonate salts (mineral sequestration). These methods are considered non-volatile because they remove carbon from the atmosphere and sequester it indefinitely and presumably for a considerable duration (thousands to millions of years).
To enhance carbon sequestration processes in oceans the following technologies have been proposed but none have achieved large scale application so far: Seaweed farming, ocean fertilisation, artificial upwelling, basalt storage, mineralization and deep sea sediments, adding bases to neutralize acids. The idea of direct deep-sea carbon dioxide injection has been abandoned.[7]
Description

Carbon sequestration is the process involved in carbon capture and the long-term storage of atmospheric carbon dioxide (CO
2)[4] and may refer specifically to:
- "The process of removing carbon from the atmosphere and depositing it in a reservoir."[9] When carried out deliberately, this may also be referred to as carbon dioxide removal, which is a form of geoengineering.
- Carbon capture and storage, where carbon dioxide is removed from flue gases (e.g., at power stations) before being stored in underground reservoirs.
- Natural biogeochemical cycling of carbon between the atmosphere and reservoirs, such as by chemical weathering of rocks.
Carbon dioxide may be captured as a pure by-product in processes related to petroleum refining or from flue gases from power generation.[10] CO
2 sequestration includes the storage part of carbon capture and storage, which refers to large-scale, artificial capture and sequestration of industrially produced CO
2 using subsurface saline aquifers, reservoirs, ocean water, aging oil fields, or other carbon sinks.
Carbon sequestration describes long-term storage of carbon dioxide or other forms of carbon to either mitigate or defer global warming and avoid dangerous climate change. It has been proposed as a way to slow the atmospheric and marine accumulation of greenhouse gases, which are released by burning fossil fuels and industrial livestock production.[11]
Carbon dioxide is naturally captured from the atmosphere through biological, chemical or physical processes. Some artificial sequestration techniques exploit these natural processes,[3] while some use entirely artificial processes.
There are three ways that this sequestration can be carried out: post-combustion capture, pre-combustion capture, and oxy-combustion.[12] A wide variety of separation techniques are being pursued, including gas phase separation, absorption into a liquid, and adsorption on a solid, as well as hybrid processes, such as adsorption/membrane systems.[13] These above processes basically capture carbon emitting from power plants, factories, fuel burning industries, and new generation livestock production facilities as they transition into restorative farming techniques to reduce carbon emissions.
In terms of carbon retention on forest land, it is better to avoid deforestation than to remove trees and subsequently reforest, as deforestation leads to irreversible effects e.g. biodiversity loss and soil degradation.[14] Additionally, the effects of af- or reforestation will be farther in the future compared to keeping existing forests intact.[15] It takes much longer − several decades − for reforested areas to return to the same carbon sequestration levels found in mature tropical forests.[16] Mackey and Dooley consider "the protection and recovery of carbon-rich and long-lived ecosystems, especially natural forests" "the major climate solution".[17] The global annual gross loss of trees is estimated to be approximately 15 billion and the global number of trees has decreased by ca 46% since the beginning of human civilization.[18]
Biological processes on land
Land-use changes that enhance natural carbon capture have the potential to capture and store large amounts of carbon dioxide each year. These include the conservation, management, and restoration of ecosystems such as forests, peatlands, wetlands, and grasslands, in addition to carbon sequestration methods in agriculture.[19]
Biosequestration

Biosequestration is the capture and storage of the atmospheric greenhouse gas carbon dioxide by continual or enhanced biological processes. This form of carbon sequestration occurs through increased rates of photosynthesis via land-use practices such as reforestation and sustainable forest management.[20][21]
Peatland
Peat bogs act as a sink for carbon because they accumulate partially decayed biomass that would otherwise continue to decay completely. There is a variance on how much the peatlands act as a carbon sink or carbon source that can be linked to varying climates in different areas of the world and different times of the year.[22] By creating new bogs, or enhancing existing ones, the amount of carbon that is sequestered by bogs would increase.[23]
Forestry
Afforestation is the establishment of a forest in an area where there was no previous tree cover. Proforestation is the practice of growing an existing forest intact toward its full ecological potential.[24] Reforestation is the replanting of trees on marginal crop and pasture lands to incorporate carbon from atmospheric CO
2 into biomass.[25][26] For this carbon sequestration process to succeed the carbon must not return to the atmosphere from mass burning or rotting when the trees die.[27] To this end, land allotted to the trees must not be converted to other uses and management of the frequency of disturbances might be necessary in order to avoid extreme events. Alternatively, the wood from them must itself be sequestered, e.g., via biochar, bio-energy with carbon storage (BECS), landfill or 'stored' by use in construction. Short of growth in perpetuity, however, reforestation with long-lived trees (>100 years) will sequester carbon for substantial periods and be released gradually, minimizing carbon's climate impact during the 21st century. Earth offers enough room to plant an additional 1.2 trillion trees.[18] Planting and protecting them would offset some 10 years of CO2 emissions and sequester 205 billion tons of carbon.[28] This approach is supported by the Trillion Tree Campaign. Restoring all degraded forests world-wide would capture about 205 billion tons of carbon in total, which is about two-thirds of all carbon emissions.[29][30]
During a 30-year period to 2050 if all new construction globally utilized 90% wood products, largely via adoption of mass timber in low rise construction, this could sequester 700 million net tons of carbon per year,[31][32] thus negating approximately 2% of annual carbon emissions as of 2019.[33] This is in addition to the elimination of carbon emissions from the displaced construction material such as steel or concrete, which are carbon-intense to produce.
Urban forestry
Urban forestry increases the amount of carbon taken up in cities by adding new tree sites and the sequestration of carbon occurs over the lifetime of the tree.[34] It is generally practiced and maintained on smaller scales, like in cities. The results of urban forestry can have different results depending on the type of vegetation that is being used, so it can function as a sink but can also function as a source of emissions.[35] In hot areas of the world, trees have an important cooling effect through shade and transpiration. This can save on the need for air conditioning which in turn can reduce GHG emissions.[35]
Wetlands
Wetland restoration involves restoring a wetland's natural biological, geological, and chemical functions through re-establishment or rehabilitation.[36] It has also been proposed as a potential climate change mitigation strategy, with carbon sequestered this way being known as blue carbon.[37] Wetland soil, particularly in coastal wetlands such as mangroves, sea grasses, and salt marshes,[37] is an important carbon reservoir; 20–30% of the world's soil carbon is found in wetlands, while only 5–8% of the world's land is composed of wetlands.[38] Studies have shown that restored wetlands can become productive CO2 sinks[39][40][41] and many restoration projects have been enacted in the US and around the world.[42][43] Aside from climate benefits, wetland restoration and conservation can help preserve biodiversity, improve water quality, and aid with flood control.[44]
As with forests, for the sequestration process to succeed, the wetland must remain undisturbed. If it is disturbed somehow, the carbon stored in the plants and sediments will be released back into the atmosphere and the ecosystem will no longer function as a carbon sink.[45] Additionally, some wetlands can release non-CO2 greenhouse gases, such as methane[46] and nitrous oxide[47] which could offset potential climate benefits. The amounts of carbon sequestered via blue carbon by wetlands can also be difficult to measure.[44]
Agriculture
Compared to natural vegetation, cropland soils are depleted in soil organic carbon (SOC). When a soil is converted from natural land or semi-natural land, such as forests, woodlands, grasslands, steppes and savannas, the SOC content in the soil reduces by about 30–40%.[48] This loss is due to the removal of plant material containing carbon, in terms of harvests. When the land use changes, the carbon in the soil will either increase or decrease, this change will continue until the soil reaches a new equilibrium. Deviations from this equilibrium can also be affected by variated climate.[49] The decreasing of SOC content can be counteracted by increasing the carbon input. This can be done with several strategies, e.g. leave harvest residues on the field, use manure as fertiliser or include perennial crops in the rotation. Perennial crops have a larger below ground biomass fraction, which increases the SOC content.[48] Perennial crops reduce the need for tillage and thus help mitigate soil erosion, and may help increase soil organic matter. Globally, soils are estimated to contain >8,580 gigatons of organic carbon, about ten times the amount in the atmosphere and much more than in vegetation.[50] Researchers have found that rising temperatures can lead to population booms in soil microbes, converting stored carbon into carbon dioxide. In laboratory experiments heating soil, fungi-rich soils released less carbon dioxide than other soils.[51]
Modification of agricultural practices is a recognized method of carbon sequestration as soil can act as an effective carbon sink offsetting as much as 20% of 2010 carbon dioxide emissions annually.[52] (See No-till farming). Restoration of organic farming and earthworms may entirely offset CO2 annual carbon excess of 4 Gt per year and drawdown the residual atmospheric excess.[53] (See Compost).
Carbon emission reduction methods in agriculture can be grouped into two categories: reducing and/or displacing emissions and enhancing carbon removal from the atmosphere. Some of these reductions involve increasing the efficiency of farm operations (e.g. more fuel-efficient equipment) while some involve interruptions in the natural carbon cycle. Also, some effective techniques (such as the elimination of stubble burning[54]) can negatively impact other environmental concerns (increased herbicide use to control weeds not destroyed by burning).
As enforcement of forest protection may not sufficiently address the drivers behind deforestation – the largest of which being the production of beef in the case of the Amazon rainforest[55] – it may also need policies. These could effectively ban and/or progressively discourage deforestation-associated trade via e.g. product information requirements, satellite monitoring like the Global Forest Watch, related eco-tariffs, and product certifications.[56][57][58]
Carbon farming
Carbon farming is a set of agricultural methods that aim to store carbon in the soil, crop roots, wood and leaves. The technical term for this is carbon sequestration. The overall goal of carbon farming is to create a net loss of carbon from the atmosphere.[59] This is done by increasing the rate at which carbon is sequestered into soil and plant material. One option is to increase the soil's organic matter content. This can also aid plant growth, improve soil water retention capacity[60] and reduce fertilizer use.[61] Sustainable forest management is another tool that is used in carbon farming.[62] Carbon farming is one component of climate-smart agriculture. It is also one of the methods for carbon dioxide removal (CDR).
Agricultural methods for carbon farming include adjusting how tillage and livestock grazing is done, using organic mulch or compost, working with biochar and terra preta, and changing the crop types. Methods used in forestry include for example reforestation and bamboo farming.Bamboo farming
Although a bamboo forest stores less total carbon than a mature forest of trees, a bamboo plantation sequesters carbon at a much faster rate than a mature forest or a tree plantation. Therefore, the farming of bamboo timber may have significant carbon sequestration potential.[63]
Deep soil
On a global basis it is estimated that soil contains about 2,500 gigatons of carbon. This is greater than 3-fold the carbon found in the atmosphere and 4-fold of that found in living plants and animals.[64] About 70% of the global soil organic carbon in non-permafrost areas is found in the deeper soil within the upper 1 meter and stabilized by mineral-organic associations.[65]
Reducing emissions
This section needs additional citations for verification. (February 2023) |
Increasing yields and efficiency generally reduces emissions as well, since more food results from the same or less effort. Techniques include more accurate use of fertilizers, less soil disturbance, better irrigation, and crop strains bred for locally beneficial traits and increased yields.[citation needed]
Replacing more energy intensive farming operations can also reduce emissions. Reduced or no-till farming requires less machine use and burns correspondingly less fuel per acre. However, no-till usually increases use of weed-control chemicals and the residue now left on the soil surface is more likely to release its CO
2 to the atmosphere as it decays, reducing the net carbon reduction.[citation needed]
In practice, most farming operations that incorporate post-harvest crop residues, wastes and byproducts back into the soil provide a carbon storage benefit.[citation needed] This is particularly the case for practices such as field burning of stubble – rather than releasing almost all of the stored CO
2 to the atmosphere, tillage incorporates the biomass back into the soil.[citation needed]
Enhancing carbon removal
All crops absorb CO
2 during growth and release it after harvest. The goal of agricultural carbon removal is to use the crop and its relation to the carbon cycle to permanently sequester carbon within the soil. This is done by selecting farming methods that return biomass to the soil and enhance the conditions in which the carbon within the plants will be reduced to its elemental nature and stored in a stable state. Methods for accomplishing this include:
- Use cover crops such as grasses and weeds as temporary cover between planting seasons
- Concentrate livestock in small paddocks for days at a time so they graze lightly but evenly. This encourages roots to grow deeper into the soil. Stock also till the soil with their hooves, grinding old grass and manures into the soil.[66]
- Cover bare paddocks with hay or dead vegetation. This protects soil from the sun and allows the soil to hold more water and be more attractive to carbon-capturing microbes.[66]
- Restore degraded, marginal, and abandoned land, which slows carbon release while returning the land to agriculture or other use.[67] Degraded land with low soil carbon pool have particularly high potential to store soil C which can be farther enhanced by proper selection of vegetation.[68][69]
Agricultural sequestration practices may have positive effects on soil, air, and water quality, be beneficial to wildlife, and expand food production. On degraded croplands, an increase of 1 ton of soil carbon pool may increase crop yield by 20 to 40 kilograms per hectare of wheat, 10 to 20 kg/ ha for maize, and 0.5 to 1 kg/ha for cowpeas.[citation needed]
The effects of soil sequestration can be reversed. If the soil is disrupted or intensive tillage practices are used, the soil becomes a net source of greenhouse gases. Typically after several decades of sequestration, soil becomes saturated and ceases to absorb carbon. This implies that there is a global limit to the amount of carbon that soil can hold.[70]
Many factors affect the costs of carbon sequestration including soil quality, transaction costs and various externalities such as leakage and unforeseen environmental damage. Because reduction of atmospheric CO
2 is a long-term concern, farmers can be reluctant to adopt more expensive agricultural techniques when there is not a clear crop, soil, or economic benefit. Governments such as Australia and New Zealand are considering allowing farmers to sell carbon credits once they document that they have sufficiently increased soil carbon content.[66][71][72][73][74][75]
Geological processes and burial

Burial
Burying biomass (such as trees)[76] directly, mimics the natural processes that created fossil fuels.[77]
Biochar burial
Biochar is charcoal created by pyrolysis of biomass waste. The resulting material is added to a landfill or used as a soil improver to create terra preta.[78][79] Addition of pyrogenic organic carbon (biochar) is a novel strategy to increase the soil-C stock for the long-term and to mitigate global-warming by offsetting the atmospheric C (up to 9.5 Gigatons C annually).[80] In the soil, the carbon is unavailable for oxidation to CO
2 and consequential atmospheric release. However concerns have been raised about biochar potentially accelerating release of the carbon already present in the soil.[81].
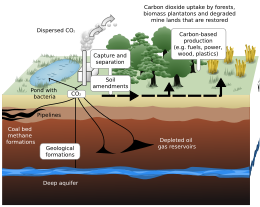
Geological sequestration
Geological sequestration refers to the storage of CO2 underground in depleted oil and gas reservoirs, saline formations, or deep, un-minable coal beds.
Once CO2 is captured from a point source, such as a cement factory,[82] it would be compressed to ≈100 bar so that it would be a supercritical fluid. In this form, the CO2 would be easy to transport via pipeline to the place of storage. The CO2 would then be injected deep underground, typically around 1 km, where it would be stable for hundreds to millions of years.[7] At these storage conditions, the density of supercritical CO2 is 600 to 800 kg / m3.[83]
The important parameters in determining a good site for carbon storage are: rock porosity, rock permeability, absence of faults, and geometry of rock layers. The medium in which the CO2 is to be stored ideally has a high porosity and permeability, such as sandstone or limestone. Sandstone can have a permeability ranging from 1 to 10−5 Darcy, and can have a porosity as high as ≈30%. The porous rock must be capped by a layer of low permeability which acts as a seal, or caprock, for the CO2. Shale is an example of a very good caprock, with a permeability of 10−5 to 10−9 Darcy. Once injected, the CO2 plume will rise via buoyant forces, since it is less dense than its surroundings. Once it encounters a caprock, it will spread laterally until it encounters a gap. If there are fault planes near the injection zone, there is a possibility the CO2 could migrate along the fault to the surface, leaking into the atmosphere, which would be potentially dangerous to life in the surrounding area. Another danger related to carbon sequestration is induced seismicity. If the injection of CO2 creates pressures that are too high underground, the formation will fracture, potentially causing an earthquake.[84]
While trapped in a rock formation, CO2 can be in the supercritical fluid phase or dissolve in groundwater/brine. It can also react with minerals in the geologic formation to precipitate carbonates. See CarbFix.
Worldwide storage capacity in oil and gas reservoirs is estimated to be 675–900 Gt CO2, and in un-minable coal seams is estimated to be 15–200 Gt CO2. Deep saline formations have the largest capacity, which is estimated to be 1,000–10,000 Gt CO2.[83] In the US, there is estimated to be at least 2,600 Gt and at most 22,000 Gt total CO2 storage capacity.[85]
There are a number of large-scale carbon capture and sequestration projects that have demonstrated the viability and safety of this method of carbon storage, which are summarized here [86] by the Global CCS Institute. The dominant monitoring technique is seismic imaging, where vibrations are generated that propagate through the subsurface. The geologic structure can be imaged from the refracted/reflected waves.[84]
The first large-scale CO
2 sequestration project which began in 1996 is called Sleipner, and is located in the North Sea where Norway's StatoilHydro strips carbon dioxide from natural gas with amine solvents and disposed of this carbon dioxide in a deep saline aquifer. In 2000, a coal-fueled synthetic natural gas plant in Beulah, North Dakota, became the world's first coal-using plant to capture and store carbon dioxide, at the Weyburn-Midale Carbon Dioxide Project.[87][needs update] Several other sequestration projects have followed. The Energy Impact Center launched the OPEN100 project in February 2020, which is the world's first open-source blueprint for the design, construction and financing of a small, standard, pressurized water reactor.[88] In September 2020, the US Department of Energy awarded $72 million in federal funding to support the development and advancement of carbon capture technologies.[89]
CO
2 has been used extensively in enhanced crude oil recovery operations in the United States beginning in 1972.[11] There are in excess of 10,000 wells that inject CO
2 in the state of Texas alone. The gas comes in part from anthropogenic sources, but is principally from large naturally occurring geologic formations of CO
2. It is transported to the oil-producing fields through a large network of over 5,000 kilometres (3,100 mi) of CO
2 pipelines. The use of CO
2 for enhanced oil recovery (EOR) methods in heavy oil reservoirs in the Western Canadian Sedimentary Basin (WCSB) has also been proposed.[90] However, transport cost remains an important hurdle. An extensive CO
2 pipeline system does not yet exist in the WCSB. Athabasca oil sands mining that produces CO
2 is hundreds of kilometers north of the subsurface Heavy crude oil reservoirs that could most benefit from CO
2 injection.
Mineral sequestration
Mineral sequestration aims to trap carbon in the form of solid carbonate salts. This process occurs slowly in nature and is responsible for the deposition and accumulation of limestone over geologic time. Carbonic acid in groundwater slowly reacts with complex silicates to dissolve calcium, magnesium, alkalis and silica and leave a residue of clay minerals. The dissolved calcium and magnesium react with bicarbonate to precipitate calcium and magnesium carbonates, a process that organisms use to make shells. When the organisms die, their shells are deposited as sediment and eventually turn into limestone. Limestones have accumulated over billions of years of geologic time and contain much of Earth's carbon. Ongoing research aims to speed up similar reactions involving alkali carbonates.[91]
Several serpentinite deposits are being investigated as potentially large scale CO2 storage sinks such as those found in NSW, Australia, where the first mineral carbonation pilot plant project is underway.[92] Beneficial re-use of magnesium carbonate from this process could provide feedstock for new products developed for the built environment and agriculture without returning the carbon into the atmosphere and so acting as a carbon sink.[93]
One proposed reaction is that of the olivine-rich rock dunite, or its hydrated equivalent serpentinite with carbon dioxide to form the carbonate mineral magnesite, plus silica and iron oxide (magnetite).
Serpentinite sequestration is favored because of the non-toxic and stable nature of magnesium carbonate. The ideal reactions involve the magnesium endmember components of the olivine (reaction 1) or serpentine (reaction 2), the latter derived from earlier olivine by hydration and silicification (reaction 3). The presence of iron in the olivine or serpentine reduces the efficiency of sequestration, since the iron components of these minerals break down to iron oxide and silica (reaction 4).
Zeolitic imidazolate frameworks
Zeolitic imidazolate frameworks is a metal-organic framework carbon dioxide sink which could be used to keep industrial emissions of carbon dioxide out of the atmosphere.[94]
Chemical processes
Developed in the Netherlands, an electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic acid;[95] This conversion uses carbon dioxide as a feedstock to generate oxalic acid.
Mineral carbonation
This section needs to be updated. (June 2019) |
CO2 exothermically reacts with metal oxides, producing stable carbonates (e.g. calcite, magnesite). This process (CO2-to-stone) occurs naturally over periods of years and is responsible for much surface limestone. Olivine is one such metal oxide.[96][self-published source?] Rocks rich in metal oxides that react with CO2, such as MgO and CaO as contained in basalts, have been proven as a viable means to achieve carbon-dioxide mineral storage.[97][98] The reaction rate can in principle be accelerated with a catalyst[99] or by increasing temperatures [dubious ] and/or pressures, or by mineral pre-treatment, although this method can require additional energy. The IPCC estimates that a power plant equipped with CCS using mineral storage would need 60–180% more energy than one without.[100] Theoretically, up to 22% of crustal mineral mass is able to form carbonates.[citation needed]
| Earthen oxide | Percent of crust | Carbonate | Enthalpy change (kJ/mol) |
|---|---|---|---|
| CaO | 4.90 | CaCO3 | −179 |
| MgO | 4.36 | MgCO3 | −118 |
| Na2O | 3.55 | Na2CO3 | −322 |
| FeO | 3.52 | FeCO3 | −85 |
| K2O | 2.80 | K2CO3 | −393.5 |
| Fe2O3 | 2.63 | FeCO3 | 112 |
| All oxides | 21.76 | All carbonates |
Ultramafic mine tailings are a readily available source of fine-grained metal oxides that could serve this purpose.[101] Accelerating passive CO2 sequestration via mineral carbonation may be achieved through microbial processes that enhance mineral dissolution and carbonate precipitation.[102][103][104]
Carbon, in the form of CO
2 can be removed from the atmosphere by chemical processes, and stored in stable carbonate mineral forms. This process (CO
2-to-stone) is known as "carbon sequestration by mineral carbonation" or mineral sequestration. The process involves reacting carbon dioxide with abundantly available metal oxides – either magnesium oxide (MgO) or calcium oxide (CaO) – to form stable carbonates. These reactions are exothermic and occur naturally (e.g., the weathering of rock over geologic time periods).[105][106]
- CaO + CO
2 → CaCO
3
- MgO + CO
2 → MgCO
3
Calcium and magnesium are found in nature typically as calcium and magnesium silicates (such as forsterite and serpentinite) and not as binary oxides. For forsterite and serpentine the reactions are:
- Mg
2SiO
4 + 2 CO
2 → 2 MgCO
3 + SiO
2
- Mg
3Si
2O
5(OH)
4+ 3 CO
2 → 3 MgCO
3 + 2 SiO
2 + 2 H
2O
The following table lists principal metal oxides of Earth's crust. Theoretically up to 22% of this mineral mass is able to form carbonates.
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
| Earthen Oxide | Percent of Crust | Carbonate | Enthalpy change (kJ/mol) |
|---|---|---|---|
| SiO 2 |
59.71 | ||
| Al 2O 3 |
15.41 | ||
| CaO | 4.90 | CaCO 3 |
−179 |
| MgO | 4.36 | MgCO 3 |
−117 |
| Na 2O |
3.55 | Na 2CO 3 |
|
| FeO | 3.52 | FeCO 3 |
|
| K 2O |
2.80 | K 2CO 3 |
|
| Fe 2O 3 |
2.63 | FeCO 3 |
|
| 21.76 | All Carbonates |
These reactions are slightly more favorable at low temperatures.[105] This process occurs naturally over geologic time frames and is responsible for much of the Earth's surface limestone. The reaction rate can be made faster however, by reacting at higher temperatures and/or pressures, although this method requires some additional energy. Alternatively, the mineral could be milled to increase its surface area, and exposed to water and constant abrasion to remove the inert Silica as could be achieved naturally by dumping Olivine in the high energy surf of beaches.[107] Experiments suggest the weathering process is reasonably quick (one year) given porous basaltic rocks.[108][109]
CO
2 naturally reacts with peridotite rock in surface exposures of ophiolites, notably in Oman. It has been suggested that this process can be enhanced to carry out natural mineralisation of CO
2.[110][111]
When CO
2 is dissolved in water and injected into hot basaltic rocks underground it has been shown that the CO
2 reacts with the basalt to form solid carbonate minerals.[112] A test plant in Iceland started up in October 2017, extracting up to 50 tons of CO2 a year from the atmosphere and storing it underground in basaltic rock.[113]
Researchers from British Columbia, developed a low cost process for the production of magnesite, also known as magnesium carbonate, which can sequester CO2 from the air, or at the point of air pollution, e.g. at a power plant. The crystals are naturally occurring, but accumulation is usually very slow.[114]
Concrete is a promising destination of captured carbon dioxide. Several advantages that concrete offers include, but not limited to: a source of plenty of calcium due to its substantial production all over the world; a thermodynamically stable condition for carbon dioxide to be stored as calcium carbonates; and its long-term capability of storing carbon dioxide as a material widely used in infrastructure.[115][116] Demolished concrete waste or recycled concrete could be also used aside from newly produced concrete.[117] Studies at HeidelbergCement show that carbon sequestration can turn those demolished and recycled concrete into a supplementary cementitious material, which acts as a secondary binder in tandem with Portland cement, for a new butch of concrete production.[118][119]
Electrochemistry
Another method uses a liquid metal catalyst and an electrolyte liquid into which CO2 is dissolved. The CO2 then converts into solid flakes of carbon. This method is done at room temperature.[120][121][122] In 2022, the team updated its work to operate at a lower temperature, and operate more quickly and with fewer steps.[123]
Industrial use
Traditional cement manufacture releases large amounts of carbon dioxide, but newly developed cement types from Novacem[124] can absorb CO
2 from ambient air during hardening.[125] A similar technique was pioneered by TecEco, which has been producing "EcoCement" since 2002.[126] Canadian startup CarbonCure Technologies takes captured CO2 and injects it into concrete as it is being mixed.[127] Carbon Upcycling UCLA is another company that uses CO
2 in concrete. Their concrete product is called CO2NCRETE, a concrete that hardens faster and is more eco-friendly than traditional concrete.[128]
In Estonia, oil shale ash, generated by power stations could be used as sorbents for CO
2 mineral sequestration. The amount of CO
2 captured averaged 60 to 65% of the carbonaceous CO
2 and 10 to 11% of the total CO
2 emissions.[129]
Chemical scrubbers
Various carbon dioxide scrubbing processes have been proposed to remove CO
2 from the air, usually using a variant of the Kraft process. Carbon dioxide scrubbing variants exist based on potassium carbonate, which can be used to create liquid fuels, or on sodium hydroxide.[130][131][132] These notably include artificial trees proposed by Klaus Lackner to remove carbon dioxide from the atmosphere using chemical scrubbers.[133][134]
Sequestration techniques in oceans
Seaweed farming
Seaweed grow in shallow and coastal areas, and capture significant amounts of carbon that can be transported to the deep ocean by oceanic mechanisms; seaweed reaching the deep ocean sequester carbon and prevent it from exchanging with the atmosphere over millennia.[135] Growing seaweed offshore with the purpose of sinking the seaweed in the depths of the sea to sequester carbon is under research.[136] In addition, seaweed grows very fast and can theoretically be harvested and processed to generate biomethane, via anaerobic digestion to generate electricity, via cogeneration/CHP or as a replacement for natural gas. One study suggested that if seaweed farms covered 9% of the ocean they could produce enough biomethane to supply Earth's equivalent demand for fossil fuel energy, remove 53 gigatonnes of CO2 per year from the atmosphere and sustainably produce 200 kg per year of fish, per person, for 10 billion people.[137] Ideal species for such farming and conversion include Laminaria digitata, Fucus serratus and Saccharina latissima.[138]
Ocean fertilisation
Ocean fertilization or ocean nourishment is a type of technology for carbon dioxide removal from the ocean based on the purposeful introduction of plant nutrients to the upper ocean to increase marine food production and to remove carbon dioxide from the atmosphere.[139][140] Ocean nutrient fertilization, for example iron fertilization, could stimulate photosynthesis in phytoplankton. The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate, some of which would sink into the deeper ocean before oxidizing. More than a dozen open-sea experiments confirmed that adding iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.[141]
This is one of the more well-researched carbon dioxide removal (CDR) approaches, however this approach would only sequester carbon on a timescale of 10-100 years dependent on ocean mixing times. While surface ocean acidity may decrease as a result of nutrient fertilization, when the sinking organic matter remineralizes, deep ocean acidity will increase. A 2021 report on CDR indicates that there is medium-high confidence that the technique could be efficient and scalable at low cost, with medium environmental risks.[142] One of the key risks of nutrient fertilization is nutrient robbing, a process by which excess nutrients used in one location for enhanced primary productivity, as in a fertilization context, are then unavailable for normal productivity downstream. This could result in ecosystem impacts far outside the original site of fertilization.[142]
A number of techniques, including fertilization by the micronutrient iron (called iron fertilization) or with nitrogen and phosphorus (both macronutrients), have been proposed. But research in the early 2020s suggested that it could only permanently sequester a small amount of carbon.[143]Artificial upwelling
Artificial upwelling or downwelling is an approach that would change the mixing layers of the ocean. Encouraging various ocean layers to mix can move nutrients and dissolved gases around, offering avenues for geoengineering.[144] Mixing may be achieved by placing large vertical pipes in the oceans to pump nutrient rich water to the surface, triggering blooms of algae, which store carbon when they grow and export carbon when they die.[144][145][146] This produces results somewhat similar to iron fertilization. One side-effect is a short-term rise in CO
2, which limits its attractiveness.[147]
Mixing layers involve transporting the denser and colder deep ocean water to the surface mixed layer. As the ocean temperature decreases with depth, more carbon dioxide and other compounds are able to dissolve in the deeper layers.[148] This can be induced by reversing the oceanic carbon cycle through the use of large vertical pipes serving as ocean pumps,[149] or a mixer array.[150] When the nutrient rich deep ocean water is moved to the surface, algae bloom occurs, resulting in a decrease in carbon dioxide due to carbon intake from phytoplankton and other photosynthetic eukaryotic organisms. The transfer of heat between the layers will also cause seawater from the mixed layer to sink and absorb more carbon dioxide. This method has not gained much traction as algae bloom harms marine ecosystems by blocking sunlight and releasing harmful toxins into the ocean.[151] The sudden increase in carbon dioxide on the surface level will also temporarily decrease the pH of the seawater, impairing the growth of coral reefs. The production of carbonic acid through the dissolution of carbon dioxide in seawater hinders marine biogenic calcification and causes major disruptions to the oceanic food chain.[152]
Basalt storage
Carbon dioxide sequestration in basalt involves the injecting of CO
2 into deep-sea formations. The CO
2 first mixes with seawater and then reacts with the basalt, both of which are alkaline-rich elements. This reaction results in the release of Ca2+ and Mg2+ ions forming stable carbonate minerals.[153]
Underwater basalt offers a good alternative to other forms of oceanic carbon storage because it has a number of trapping measures to ensure added protection against leakage. These measures include "geochemical, sediment, gravitational and hydrate formation." Because CO
2 hydrate is denser than CO
2 in seawater, the risk of leakage is minimal. Injecting the CO
2 at depths greater than 2,700 meters (8,900 ft) ensures that the CO
2 has a greater density than seawater, causing it to sink.[154]
One possible injection site is Juan de Fuca plate. Researchers at the Lamont–Doherty Earth Observatory found that this plate at the western coast of the United States has a possible storage capacity of 208 gigatons. This could cover the entire current U.S. carbon emissions for over 100 years.[154]
This process is undergoing tests as part of the CarbFix project, resulting in 95% of the injected 250 tonnes of CO2 to solidify into calcite in two years, using 25 tonnes of water per tonne of CO2.[109][155]
Mineralization and deep sea sediments
Similar to mineralization processes that take place within rocks, mineralization can also occur under the sea. The rate of dissolution of carbon dioxide from atmosphere to oceanic regions is determined by the circulation period of the ocean and buffering ability of subducting surface water.[156] Researchers have demonstrated that the carbon dioxide marine storage at several kilometers depth could be viable for up to 500 years, but is dependent on injection site and conditions. Several studies have shown that although it may fix carbon dioxide effectively, carbon dioxide may be released back to the atmosphere over time. However, this is unlikely for at least a few more centuries. The neutralization of CaCO3, or balancing the concentration of CaCO3 on the seafloor, land and in the ocean, can be measured on a timescale of thousands of years. More specifically, the predicted time is 1700 years for ocean and approximately 5000 to 6000 years for land.[157][158] Further, the dissolution time for CaCO3 can be improved by injecting near or downstream of the storage site.[159]
In addition to carbon mineralization, another proposal is deep sea sediment injection. It injects liquid carbon dioxide at least 3000 m below the surface directly into ocean sediments to generate carbon dioxide hydrate. Two regions are defined for exploration: 1) the negative buoyancy zone (NBZ), which is the region between liquid carbon dioxide denser than surrounding water and where liquid carbon dioxide has neutral buoyancy, and 2) the hydrate formation zone (HFZ), which typically has low temperatures and high pressures. Several research models have shown that the optimal depth of injection requires consideration of intrinsic permeability and any changes in liquid carbon dioxide permeability for optimal storage. The formation of hydrates decreases liquid carbon dioxide permeability, and injection below HFZ is more energetically favored than within the HFZ. If the NBZ is a greater column of water than the HFZ, the injection should happen below the HFZ and directly to the NBZ. In this case, liquid carbon dioxide will sink to the NBZ and be stored below the buoyancy and hydrate cap. Carbon dioxide leakage can occur if there is dissolution into pore fluid or via molecular diffusion. However, this occurs over thousands of years.[159][160][161]
Adding bases to neutralize acids
Carbon dioxide forms carbonic acid when dissolved in water, so ocean acidification is a significant consequence of elevated carbon dioxide levels, and limits the rate at which it can be absorbed into the ocean (the solubility pump). A variety of different bases have been suggested that could neutralize the acid and thus increase CO
2 absorption.[162][163][164][165][166] For example, adding crushed limestone to oceans enhances the absorption of carbon dioxide.[167] Another approach is to add sodium hydroxide to oceans which is produced by electrolysis of salt water or brine, while eliminating the waste hydrochloric acid by reaction with a volcanic silicate rock such as enstatite, effectively increasing the rate of natural weathering of these rocks to restore ocean pH.[168][169][170]
Single-step carbon sequestration and storage
Single-step carbon sequestration and storage is a saline water-based mineralization technology extracting carbon dioxide from seawater and storing it in the form of solid minerals.[171]
Abandoned ideas
Direct deep-sea carbon dioxide injection
It was once suggested that CO2 could be stored in the oceans by direct injection into the deep ocean and storing it there for some centuries. At the time, this proposal was called "ocean storage" but more precisely it was known as "direct deep-sea carbon dioxide injection". However, the interest in this avenue of carbon storage has much reduced since about 2001 because of concerns about the unknown impacts on marine life[172]: 279 , high costs and concerns about its stability or permanence.[7] The "IPCC Special Report on Carbon Dioxide Capture and Storage" in 2005 did include this technology as an option.[172]: 279 However, the IPCC Fifth Assessment Report in 2014 no longer mentioned the term "ocean storage" in its report on climate change mitigation methods.[173] The most recent IPCC Sixth Assessment Report in 2022 also no longer includes any mention of "ocean storage" in its "Carbon Dioxide Removal taxonomy".[174]: 12–37
Cost
Cost of the sequestration (not including capture and transport) varies but is below US$10 per tonne in some cases where onshore storage is available.[175] For example Carbfix cost is around US$25 per tonne of CO2.[176] A 2020 report estimated sequestration in forests (so including capture) at US$35 for small quantities to US$280 per tonne for 10% of the total required to keep to 1.5 C warming.[177] But there is risk of forest fires releasing the carbon.[178]
Researchers have raised the concern that the use of carbon offsets – such as by maintaining forests, reforestation or carbon capture – as well as renewable energy certificates[179] allow polluting companies a business-as-usual approach to continue releasing greenhouse gases[180][181] and for being, inappropriately trusted, untried techno-fixes.[182] This also includes the 2022 IPCC report on climate change criticized for containing "a lot of pipe dreams", relying on large negative emissions technologies.[183] A review of studies by the Stanford Solutions Project concluded that relying on Carbon capture and storage/utilization (CCS/U) is a dangerous distraction, with it (in most and large-scale cases) being expensive, increasing air pollution and mining, inefficient and unlikely to be deployable at the scale required in time.[184]

Applications in climate change policies
United States
Starting in the mid-late 2010s, many pieces of US climate and environment policy have sought to make use of the climate change mitigation potential of carbon sequestration. Many of these policies involve either conservation of carbon sink ecosystems, such as forests and wetlands, or encouraging agricultural and land use practices designed to increase carbon sequestration such as carbon farming or agroforestry, often through financial incentivization for farmers and landowners.
The Executive Order on Tackling the Climate Crisis at Home and Abroad, signed by president Joe Biden on January 27, 2021, includes several mentions of carbon sequestration via conservation and restoration of carbon sink ecosystems, such as wetlands and forests. These include emphasizing the importance of farmers, landowners, and coastal communities in carbon sequestration, directing the Treasury Department to promote conservation of carbon sinks through market based mechanisms, and directing the Department of the Interior to collaborate with other agencies to create a Civilian Climate Corps to increase carbon sequestration in agriculture, among other things.[185]
See also
- Bio-energy with carbon capture and storage
- Blue carbon
- Carbon storage in the North Sea
- Repurposing offshore drilling rigs for storing carbon
References
- ^ "CCS Explained". UKCCSRC. Archived from the original on June 28, 2020. Retrieved June 27, 2020.
- ^ IPCC (2021). Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S. L.; et al. (eds.). Climate Change 2021: The Physical Science Basis (PDF). Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press (In Press).
- ^ a b c "Energy Terms Glossary S". Nebraska Energy Office. Archived from the original on May 27, 2010. Retrieved May 9, 2010.
- ^ a b Sedjo, Roger; Sohngen, Brent (2012). "Carbon Sequestration in Forests and Soils". Annual Review of Resource Economics. 4: 127–144. doi:10.1146/annurev-resource-083110-115941.
- ^ "Geoengineering the climate: science, governance and uncertainty". The Royal Society. 2009. Archived from the original on September 8, 2011. Retrieved September 10, 2011.
- ^ Myles, Allen (September 2020). "The Oxford Principles for Net Zero Aligned Carbon Offsetting" (PDF). Archived (PDF) from the original on October 2, 2020. Retrieved December 10, 2021.
- ^ a b c Benson, S.M.; Surles, T. (October 1, 2006). "Carbon Dioxide Capture and Storage: An Overview With Emphasis on Capture and Storage in Deep Geological Formations". Proceedings of the IEEE. 94 (10): 1795–1805. doi:10.1109/JPROC.2006.883718. ISSN 0018-9219. S2CID 27994746. Archived from the original on June 11, 2020. Retrieved September 10, 2019.
- ^ Abdulla, Ahmed; Hanna, Ryan; Schell, Kristen R.; Babacan, Oytun; et al. (December 29, 2021). "Explaining successful and failed investments in U.S. carbon capture and storage using empirical and expert assessments". Environmental Research Letters. 16 (1): 014036. Bibcode:2021ERL....16a4036A. doi:10.1088/1748-9326/abd19e.
- ^ "Glossary of climate change acronyms". United Nations Framework Convention on Climate Change. Archived from the original on March 30, 2018. Retrieved July 15, 2010.
- ^ "Alberta producers rewarded for use of CO2 in enhanced oil recovery". PointCarbon. May 25, 2004. Archived from the original on May 6, 2008. Retrieved August 21, 2015.
- ^ a b Hodrien, Chris (October 24, 2008). Squaring the Circle on Coal – Carbon Capture and Storage. Claverton Energy Group Conference, Bath. Archived from the original (PDF) on May 31, 2009. Retrieved May 9, 2010.
- ^ Kanniche, Mohamed; Gros-Bonnivard, René; Jaud, Philippe; Valle-Marcos, Jose; Amann, Jean-Marc; Bouallou, Chakib (January 1, 2010). "Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture". Applied Thermal Engineering. Selected Papers from the 11th Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction. 30 (1): 53–62. doi:10.1016/j.applthermaleng.2009.05.005. ISSN 1359-4311.
- ^ Badiei, Marzieh; Asim, Nilofar; Yarmo, Mohd Ambar; Jahim, Jamaliah Md; Sopian, Kamaruzzaman (2012). "Overview of Carbon Dioxide Separation Technology". Power and Energy Systems and Applications. Las Vegas, USA: ACTAPRESS. doi:10.2316/P.2012.788-067. ISBN 978-0-88986-939-4.
- ^ "Press corner". European Commission – European Commission. Retrieved September 28, 2020.
- ^ "Why Keeping Mature Forests Intact Is Key to the Climate Fight". Yale E360. Retrieved September 28, 2020.
- ^ "Would a Large-scale Reforestation Effort Help Counter the Global Warming Impacts of Deforestation?". Union of Concerned Scientists. September 1, 2012. Retrieved September 28, 2020.
- ^ "Planting trees is no substitute for natural forests". phys.org. Retrieved May 2, 2021.
- ^ a b Crowther, T. W.; Glick, H. B.; Covey, K. R.; Bettigole, C.; Maynard, D. S.; Thomas, S. M.; Smith, J. R.; Hintler, G.; Duguid, M. C.; Amatulli, G.; Tuanmu, M.-N.; Jetz, W.; Salas, C.; Stam, C.; Piotto, D. (September 2015). "Mapping tree density at a global scale". Nature. 525 (7568): 201–205. Bibcode:2015Natur.525..201C. doi:10.1038/nature14967. ISSN 1476-4687. PMID 26331545. S2CID 4464317.
- ^ *IPCC (2022). "Summary for Policymakers" (PDF). Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.
- ^ Beerling, David (2008). The Emerald Planet: How Plants Changed Earth's History. Oxford University Press. pp. 194–5. ISBN 978-0-19-954814-9.
- ^ National Academies Of Sciences, Engineering (2019). Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Washington, D.C.: National Academies of Sciences, Engineering, and Medicine. pp. 45–136. doi:10.17226/25259. ISBN 978-0-309-48452-7. PMID 31120708. S2CID 134196575.
- ^ Strack, Maria, ed. (2008). Peatlands and climate change. Calgary: University of Calgary. pp. 13–23. ISBN 978-952-99401-1-0.
- ^ Lovett, Richard (May 3, 2008). "Burying biomass to fight climate change". New Scientist (2654). Archived from the original on December 31, 2010. Retrieved May 9, 2010.
- ^ Moomaw, William R.; Masino, Susan A.; Faison, Edward K. (2019). "Intact Forests in the United States: Proforestation Mitigates Climate Change and Serves the Greatest Good". Frontiers in Forests and Global Change. 2: 27. doi:10.3389/ffgc.2019.00027. ISSN 2624-893X.
- ^ McDermott, Matthew (August 22, 2008). "Can Aerial Reforestation Help Slow Climate Change? Discovery Project Earth Examines Re-Engineering the Planet's Possibilities". TreeHugger. Archived from the original on March 30, 2010. Retrieved May 9, 2010.
- ^ Lefebvre, David; Williams, Adrian G.; Kirk, Guy J. D.; Paul; Burgess, J.; Meersmans, Jeroen; Silman, Miles R.; Román-Dañobeytia, Francisco; Farfan, Jhon; Smith, Pete (October 7, 2021). "Assessing the carbon capture potential of a reforestation project". Scientific Reports. 11 (1): 19907. Bibcode:2021NatSR..1119907L. doi:10.1038/s41598-021-99395-6. ISSN 2045-2322. PMC 8497602. PMID 34620924.
- ^ Gorte, Ross W. (2009). Carbon Sequestration in Forests (PDF) (RL31432 ed.). Congressional Research Service.
- ^ Bastin, Jean-Francois; Finegold, Yelena; Garcia, Claude; Mollicone, Danilo; Rezende, Marcelo; Routh, Devin; Zohner, Constantin M.; Crowther, Thomas W. (July 5, 2019). "The global tree restoration potential". Science. 365 (6448): 76–79. Bibcode:2019Sci...365...76B. doi:10.1126/science.aax0848. PMID 31273120. S2CID 195804232.
- ^ Tutton, Mark (July 4, 2019). "Restoring forests could capture two-thirds of the carbon humans have added to the atmosphere". CNN. Archived from the original on March 23, 2020. Retrieved January 23, 2020.
- ^ Chazdon, Robin; Brancalion, Pedro (July 5, 2019). "Restoring forests as a means to many ends". Science. 365 (6448): 24–25. Bibcode:2019Sci...365...24C. doi:10.1126/science.aax9539. PMID 31273109. S2CID 195804244.
- ^ Toussaint, Kristin (January 27, 2020). "Building with timber instead of steel could help pull millions of tons of carbon from the atmosphere". Fast Company. Archived from the original on January 28, 2020. Retrieved January 29, 2020.
- ^ Churkina, Galina; Organschi, Alan; Reyer, Christopher P. O.; Ruff, Andrew; Vinke, Kira; Liu, Zhu; Reck, Barbara K.; Graedel, T. E.; Schellnhuber, Hans Joachim (January 27, 2020). "Buildings as a global carbon sink". Nature Sustainability. 3 (4): 269–276. doi:10.1038/s41893-019-0462-4. ISSN 2398-9629. S2CID 213032074. Archived from the original on January 28, 2020. Retrieved January 29, 2020.
- ^ "Annual CO2 emissions worldwide 2019". Statista. Archived from the original on February 22, 2021. Retrieved March 11, 2021.
- ^ McPherson, E. Gregory; Xiao, Qingfu; Aguaron, Elena (December 1, 2013). "A new approach to quantify and map carbon stored, sequestered and emissions avoided by urban forests". Landscape and Urban Planning. 120: 70–84. doi:10.1016/j.landurbplan.2013.08.005. ISSN 0169-2046.
- ^ a b Velasco, Erik; Roth, Matthias; Norford, Leslie; Molina, Luisa T. (April 2016). "Does urban vegetation enhance carbon sequestration?". Landscape and Urban Planning. 148: 99–107. doi:10.1016/j.landurbplan.2015.12.003.
- ^ US EPA, OW (July 27, 2018). "Basic Information about Wetland Restoration and Protection". US EPA. Archived from the original on April 28, 2021. Retrieved April 28, 2021.
- ^ a b US Department of Commerce, National Oceanic and Atmospheric Administration. "What is Blue Carbon?". oceanservice.noaa.gov. Archived from the original on April 22, 2021. Retrieved April 28, 2021.
- ^ Mitsch, William J.; Bernal, Blanca; Nahlik, Amanda M.; Mander, Ülo; Zhang, Li; Anderson, Christopher J.; Jørgensen, Sven E.; Brix, Hans (April 1, 2013). "Wetlands, carbon, and climate change". Landscape Ecology. 28 (4): 583–597. doi:10.1007/s10980-012-9758-8. ISSN 1572-9761. S2CID 11939685. Archived from the original on November 22, 2021. Retrieved April 28, 2021.
- ^ Valach, Alex C.; Kasak, Kuno; Hemes, Kyle S.; Anthony, Tyler L.; Dronova, Iryna; Taddeo, Sophie; Silver, Whendee L.; Szutu, Daphne; Verfaillie, Joseph; Baldocchi, Dennis D. (March 25, 2021). "Productive wetlands restored for carbon sequestration quickly become net CO2 sinks with site-level factors driving uptake variability". PLOS ONE. 16 (3): e0248398. Bibcode:2021PLoSO..1648398V. doi:10.1371/journal.pone.0248398. ISSN 1932-6203. PMC 7993764. PMID 33765085.
- ^ Bu, Xiaoyan; Cui, Dan; Dong, Suocheng; Mi, Wenbao; Li, Yu; Li, Zhigang; Feng, Yaliang (January 2020). "Effects of Wetland Restoration and Conservation Projects on Soil Carbon Sequestration in the Ningxia Basin of the Yellow River in China from 2000 to 2015". Sustainability. 12 (24): 10284. doi:10.3390/su122410284.
- ^ Badiou, Pascal; McDougal, Rhonda; Pennock, Dan; Clark, Bob (June 1, 2011). "Greenhouse gas emissions and carbon sequestration potential in restored wetlands of the Canadian prairie pothole region". Wetlands Ecology and Management. 19 (3): 237–256. doi:10.1007/s11273-011-9214-6. ISSN 1572-9834. S2CID 30476076.
- ^ "Restoring Wetlands - Wetlands (U.S. National Park Service)". www.nps.gov. Archived from the original on April 28, 2021. Retrieved April 28, 2021.
- ^ "A new partnership for wetland restoration | ICPDR – International Commission for the Protection of the Danube River". www.icpdr.org. Archived from the original on April 28, 2021. Retrieved April 28, 2021.
- ^ a b "Fact Sheet: Blue Carbon". American University. Archived from the original on April 28, 2021. Retrieved April 28, 2021.
- ^ "Carbon Sequestration in Wetlands | MN Board of Water, Soil Resources". bwsr.state.mn.us. Archived from the original on April 28, 2021. Retrieved April 28, 2021.
- ^ Bridgham, Scott D.; Cadillo-Quiroz, Hinsby; Keller, Jason K.; Zhuang, Qianlai (May 2013). "Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales". Global Change Biology. 19 (5): 1325–1346. Bibcode:2013GCBio..19.1325B. doi:10.1111/gcb.12131. PMID 23505021. S2CID 14228726.
- ^ Thomson, Andrew J.; Giannopoulos, Georgios; Pretty, Jules; Baggs, Elizabeth M.; Richardson, David J. (May 5, 2012). "Biological sources and sinks of nitrous oxide and strategies to mitigate emissions". Philosophical Transactions of the Royal Society B: Biological Sciences. 367 (1593): 1157–1168. doi:10.1098/rstb.2011.0415. ISSN 0962-8436. PMC 3306631. PMID 22451101.
- ^ a b Poeplau, Christopher; Don, Axel (February 1, 2015). "Carbon sequestration in agricultural soils via cultivation of cover crops – A meta-analysis". Agriculture, Ecosystems & Environment. 200 (Supplement C): 33–41. doi:10.1016/j.agee.2014.10.024.
- ^ Goglio, Pietro; Smith, Ward N.; Grant, Brian B.; Desjardins, Raymond L.; McConkey, Brian G.; Campbell, Con A.; Nemecek, Thomas (October 1, 2015). "Accounting for soil carbon changes in agricultural life cycle assessment (LCA): a review". Journal of Cleaner Production. 104: 23–39. doi:10.1016/j.jclepro.2015.05.040. ISSN 0959-6526. Archived from the original on October 30, 2020. Retrieved November 27, 2017.
- ^ Blakemore, R.J. (November 2018). "Non-flat Earth Recalibrated for Terrain and Topsoil". Soil Systems. 2 (4): 64. doi:10.3390/soilsystems2040064.
- ^ Kreier, Freda (November 30, 2021). "Fungi may be crucial to storing carbon in soil as the Earth warms". Science News. Archived from the original on November 30, 2021. Retrieved December 1, 2021.
- ^ Biggers, Jeff (November 20, 2015). "Iowa's Climate-Change Wisdom". New York Times. Archived from the original on November 23, 2015. Retrieved November 21, 2015.
- ^ VermEcology (November 11, 2019). "Earthworm Cast Carbon Storage". Archived from the original on November 12, 2019. Retrieved November 12, 2019.
- ^ "The Burning Problem". The Nature Conservancy. Retrieved January 19, 2023.
- ^ Santos, Alex Mota dos; Silva, Carlos Fabricio Assunção da; Almeida Junior, Pedro Monteiro de; Rudke, Anderson Paulo; Melo, Silas Nogueira de (September 15, 2021). "Deforestation drivers in the Brazilian Amazon: assessing new spatial predictors". Journal of Environmental Management. 294: 113020. doi:10.1016/j.jenvman.2021.113020. ISSN 0301-4797.
- ^ Siegle, Lucy (August 9, 2015). "Has the Amazon rainforest been saved, or should I still worry about it?". The Guardian. Retrieved October 21, 2015.
- ^ Henders, Sabine; Persson, U Martin; Kastner, Thomas (December 1, 2015). "Trading forests: land-use change and carbon emissions embodied in production and exports of forest-risk commodities". Environmental Research Letters. 10 (12): 125012. Bibcode:2015ERL....10l5012H. doi:10.1088/1748-9326/10/12/125012.
- ^ Kehoe, Laura; dos Reis, Tiago N. P.; Meyfroidt, Patrick; Bager, Simon; Seppelt, Ralf; Kuemmerle, Tobias; Berenguer, Erika; Clark, Michael; Davis, Kyle Frankel; zu Ermgassen, Erasmus K. H. J.; Farrell, Katharine Nora; Friis, Cecilie; Haberl, Helmut; Kastner, Thomas; Murtough, Katie L.; Persson, U. Martin; Romero-Muñoz, Alfredo; O’Connell, Chris; Schäfer, Viola Valeska; Virah-Sawmy, Malika; le Polain de Waroux, Yann; Kiesecker, Joseph (September 18, 2020). "Inclusion, Transparency, and Enforcement: How the EU-Mercosur Trade Agreement Fails the Sustainability Test". One Earth. 3 (3): 268–272. Bibcode:2020OEart...3..268K. doi:10.1016/j.oneear.2020.08.013. ISSN 2590-3322. S2CID 224906100.
- ^ Nath, Arun Jyoti; Lal, Rattan; Das, Ashesh Kumar (January 1, 2015). "Managing woody bamboos for carbon farming and carbon trading". Global Ecology and Conservation. 3: 654–663. doi:10.1016/j.gecco.2015.03.002. ISSN 2351-9894.
- ^ "Carbon Farming | Carbon Cycle Institute". www.carboncycle.org. Archived from the original on May 21, 2021. Retrieved April 27, 2018.
- ^ Almaraz, Maya; Wong, Michelle Y.; Geoghegan, Emily K.; Houlton, Benjamin Z. (2021). "A review of carbon farming impacts on nitrogen cycling, retention, and loss". Annals of the New York Academy of Sciences. 1505 (1): 102–117. doi:10.1111/nyas.14690. ISSN 0077-8923. S2CID 238202676.
- ^ Jindal, Rohit; Swallow, Brent; Kerr, John (2008). "Forestry-based carbon sequestration projects in Africa: Potential benefits and challenges". Natural Resources Forum. 32 (2): 116–130. doi:10.1111/j.1477-8947.2008.00176.x. ISSN 1477-8947.
- ^ Devi, Angom Sarjubala; Singh, Kshetrimayum Suresh (January 12, 2021). "Carbon storage and sequestration potential in aboveground biomass of bamboos in North East India". Scientific Reports. 11 (1): 837. doi:10.1038/s41598-020-80887-w. ISSN 2045-2322. PMC 7803772. PMID 33437001.
- ^ "Soil carbon: what we've learned so far". Cawood. Retrieved January 20, 2023.
- ^ Georgiou, Katerina; Jackson, Robert B.; Vindušková, Olga; Abramoff, Rose Z.; Ahlström, Anders; Feng, Wenting; Harden, Jennifer W.; Pellegrini, Adam F. A.; Polley, H. Wayne; Soong, Jennifer L.; Riley, William J.; Torn, Margaret S. (July 1, 2022). "Global stocks and capacity of mineral-associated soil organic carbon". Nature Communications. 13 (1): 3797. doi:10.1038/s41467-022-31540-9. ISSN 2041-1723. PMC 9249731. PMID 35778395.
- ^ a b c "FACTBOX: Carbon farming on rise in Australia". Reuters. June 16, 2009. Archived from the original on November 22, 2021. Retrieved May 9, 2010.
- ^ Bell, Stephen M.; Barriocanal, Carles; Terrer, César; Rosell-Melé, Antoni (June 1, 2020). "Management opportunities for soil carbon sequestration following agricultural land abandonment". Environmental Science & Policy. 108: 104–111. doi:10.1016/j.envsci.2020.03.018. ISSN 1462-9011. S2CID 218795674.
- ^ Vindušková, Olga; Frouz, Jan (July 1, 2013). "Soil carbon accumulation after open-cast coal and oil shale mining in Northern Hemisphere: a quantitative review". Environmental Earth Sciences. 69 (5): 1685–1698. Bibcode:2013EES....69.1685V. doi:10.1007/s12665-012-2004-5. ISSN 1866-6299. S2CID 129185046. Archived from the original on November 22, 2021. Retrieved July 2, 2021.
- ^ Frouz, Jan; Livečková, Miluše; Albrechtová, Jana; Chroňáková, Alica; Cajthaml, Tomáš; Pižl, Václav; Háněl, Ladislav; Starý, Josef; Baldrian, Petr; Lhotáková, Zuzana; Šimáčková, Hana; Cepáková, Šárka (December 1, 2013). "Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites". Forest Ecology and Management. 309: 87–95. doi:10.1016/j.foreco.2013.02.013. ISSN 0378-1127. Archived from the original on July 9, 2021. Retrieved July 2, 2021.
- ^ Sundermeiera, A.P.; Islam, K.R.; Raut, Y.; Reeder, R.C.; Dick, W.A. (September 2010). "Continuous No-Till Impacts on Soil Biophysical Carbon Sequestration". Soil Science Society of America Journal. 75 (5): 1779–1788. Bibcode:2011SSASJ..75.1779S. doi:10.2136/sssaj2010.0334.
- ^ Smith, Pete; Martino, Daniel; Cai, Zucong; et al. (February 2008). "Greenhouse gas mitigation in agriculture". Philosophical Transactions of the Royal Society B. 363 (1492): 789–813. doi:10.1098/rstb.2007.2184. PMC 2610110. PMID 17827109..
- ^ "Environmental Co Benefits of Sequestration Practices. 2006. June 1, 2009". Archived from the original on May 11, 2009.
- ^ Lal, R. (June 11, 2004). "Soil Carbon Sequestration Impacts on Global Climate Change and Food Security". Science. 304 (5677): 1623–1627. Bibcode:2004Sci...304.1623L. doi:10.1126/science.1097396. PMID 15192216. S2CID 8574723.
- ^ "Addressing Reversibility (Duration) for Projects". US Environmental Protection Agency. 2006. June 1, 2009. Archived from the original on October 13, 2008.
- ^ Renwick, A.; Ball, A.; Pretty, J.N. (August 2002). "Biological and Policy Constraints on the Adoption of Carbon Farming in Temperate Regions". Philosophical Transactions of the Royal Society A. 360 (1797): 1721–40. Bibcode:2002RSPTA.360.1721R. doi:10.1098/rsta.2002.1028. PMID 12460494. S2CID 41627741. pp. 1722, 1726–29.
- ^ Zeng, Ning (2008). "Carbon sequestration via wood burial". Carbon Balance and Management. 3 (1): 1. doi:10.1186/1750-0680-3-1. PMC 2266747. PMID 18173850.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lovett, Richard (May 3, 2008). "Burying biomass to fight climate change". New Scientist (2654). Archived from the original on August 3, 2009. Retrieved May 9, 2010.
- ^ Lehmann, J.; Gaunt, J.; Rondon, M. (2006). "Bio-char sequestration in terrestrial ecosystems – a review" (PDF). Mitigation and Adaptation Strategies for Global Change (Submitted manuscript). 11 (2): 403–427. CiteSeerX 10.1.1.183.1147. doi:10.1007/s11027-005-9006-5. S2CID 4696862. Archived (PDF) from the original on October 25, 2018. Retrieved July 31, 2018.
- ^ "International Biochar Initiative | International Biochar Initiative". Biochar-international.org. Archived from the original on May 5, 2012. Retrieved May 9, 2010.
- ^ Yousaf, Balal; Liu, Guijian; Wang, Ruwei; Abbas, Qumber; Imtiaz, Muhammad; Liu, Ruijia (2016). "Investigating the biochar effects on C-mineralization and sequestration of carbon in soil compared with conventional amendments using stable isotope (δ13C) approach". GCB Bioenergy. 9 (6): 1085–1099. doi:10.1111/gcbb.12401.
- ^ Wardle, David A.; Nilsson, Marie-Charlotte; Zackrisson, Olle (May 2, 2008). "Fire-Derived Charcoal Causes Loss of Forest Humus". Science. 320 (5876): 629. Bibcode:2008Sci...320..629W. doi:10.1126/science.1154960. ISSN 0036-8075. PMID 18451294. S2CID 22192832. Archived from the original on August 8, 2021. Retrieved August 8, 2021.
- ^ Morgan, Sam (September 6, 2019). "Norway's carbon storage project boosted by European industry". www.euractiv.com. Archived from the original on June 27, 2020. Retrieved June 27, 2020.
- ^ a b Aydin, Gokhan; Karakurt, Izzet; Aydiner, Kerim (September 1, 2010). "Evaluation of geologic storage options of CO2: Applicability, cost, storage capacity and safety". Energy Policy. Special Section on Carbon Emissions and Carbon Management in Cities with Regular Papers. 38 (9): 5072–5080. doi:10.1016/j.enpol.2010.04.035.
- ^ a b Smit, Berend; Reimer, Jeffrey A.; Oldenburg, Curtis M.; Bourg, Ian C. (2014). Introduction to Carbon Capture and Sequestration. London: Imperial College Press. ISBN 978-1783263288.
- ^ "NETL's 2015 Carbon Storage Atlas Shows Increase in U.S. CO2 Storage Potential". Archived from the original on September 26, 2021. Retrieved September 26, 2021.
- ^ "Large-scale CCS facilities". www.globalccsinstitute.com. Global Carbon Capture and Storage Institute. Archived from the original on May 13, 2016. Retrieved May 7, 2016.
- ^ "Weyburn-Midale CO
2 Project, World's first CO
2 measuring, monitoring and verification initiative". Petroleum Technology Research Centre. Archived from the original on February 17, 2007. Retrieved April 9, 2009. - ^ "Last Energy raises $3 million to fight climate change with nuclear energy". VentureBeat. February 25, 2020. Archived from the original on January 12, 2021. Retrieved December 16, 2020.
- ^ "Department of Energy Invests $72 Million in Carbon Capture Technologies". Energy.gov. Archived from the original on November 27, 2020. Retrieved December 16, 2020.
- ^ "Subscription Verification". Dailyoilbulletin.com. Retrieved May 9, 2010.[dead link]
- ^ "Carbon-capture Technology To Help UK Tackle Global Warming". ScienceDaily. July 27, 2007.
- ^ "Mineral carbonation project for NSW". June 9, 2010.
- ^ Frost, B. R.; Beard, J. S. (April 3, 2007). "On Silica Activity and Serpentinization". Journal of Petrology. 48 (7): 1351–1368. doi:10.1093/petrology/egm021.
- ^ "New materials can selectively capture CO2, scientists say". CBC News. February 15, 2008.
- ^ Bouwman, Elisabeth; Angamuthu, Raja; Byers, Philip; Lutz, Martin; Spek, Anthony L. (July 15, 2010). "Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex". Science. 327 (5393): 313–315. Bibcode:2010Sci...327..313A. CiteSeerX 10.1.1.1009.2076. doi:10.1126/science.1177981. PMID 20075248. S2CID 24938351.
- ^ Schuiling, Olaf. "Olaf Schuiling proposes olivine rock grinding". Archived from the original on April 11, 2013. Retrieved December 23, 2011.
- ^ Snæbjörnsdóttir, Sandra Ó.; Sigfússon, Bergur; Marieni, Chiara; Goldberg, David; Gislason, Sigurður R.; Oelkers, Eric H. (2020). "Carbon dioxide storage through mineral carbonation" (PDF). Nature Reviews Earth & Environment. 1 (2): 90–102. Bibcode:2020NRvEE...1...90S. doi:10.1038/s43017-019-0011-8. S2CID 210716072.
- ^ McGrail, B. Peter; et al. (2014). "Injection and Monitoring at the Wallula Basalt Pilot Project". Energy Procedia. 63: 2939–2948. doi:10.1016/j.egypro.2014.11.316.
- ^ Bhaduri, Gaurav A.; Šiller, Lidija (2013). "Nickel nanoparticles catalyse reversible hydration of CO2 for mineralization carbon capture and storage". Catalysis Science & Technology. 3 (5): 1234. doi:10.1039/C3CY20791A.
- ^ [IPCC, 2005] IPCC special report on CO2 Capture and Storage. Prepared by working group III of the Intergovernmental Panel on Climate Change. Metz, B., O. Davidson, H. C. de Coninck, M. Loos, and L.A. Meyer (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 442 pp. Available in full at www.ipcc.ch Archived 10 February 2010 at the Wayback Machine (PDF - 22.8MB)
- ^ Wilson, Siobhan A.; Dipple, Gregory M.; Power, Ian M.; Thom, James M.; Anderson, Robert G.; Raudsepp, Mati; Gabites, Janet E.; Southam, Gordon (2009). "CO2 Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada". Economic Geology. 104: 95–112. doi:10.2113/gsecongeo.104.1.95.
- ^ Power, Ian M.; Dipple, Gregory M.; Southam, Gordon (2010). "Bioleaching of Ultramafic Tailings by Acidithiobacillus spp. For CO2 Sequestration". Environmental Science & Technology. 44 (1): 456–62. Bibcode:2010EnST...44..456P. doi:10.1021/es900986n. PMID 19950896.
- ^ Power, Ian M; Wilson, Siobhan A; Thom, James M; Dipple, Gregory M; Southam, Gordon (2007). "Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada". Geochemical Transactions. 8: 13. doi:10.1186/1467-4866-8-13. PMC 2213640. PMID 18053262.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Power, Ian M.; Wilson, Siobhan A.; Small, Darcy P.; Dipple, Gregory M.; Wan, Wankei; Southam, Gordon (2011). "Microbially Mediated Mineral Carbonation: Roles of Phototrophy and Heterotrophy". Environmental Science & Technology. 45 (20): 9061–8. Bibcode:2011EnST...45.9061P. doi:10.1021/es201648g. PMID 21879741.
- ^ a b Herzog, Howard (March 14, 2002). "Carbon Sequestration via Mineral Carbonation: Overview and Assessment" (PDF). Massachusetts Institute of Technology. Archived (PDF) from the original on May 17, 2008. Retrieved March 5, 2009.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Conference Proceedings". netl.doe.gov. Retrieved December 30, 2021.
- ^ Schuiling, R.D.; Boer, de P.L. (2011). "Rolling stones; fast weathering of olivine in shallow seas for cost-effective CO2 capture and mitigation of global warming and ocean acidification" (PDF). Earth System Dynamics Discussions. 2 (2): 551–568. Bibcode:2011ESDD....2..551S. doi:10.5194/esdd-2-551-2011. hdl:1874/251745. Archived (PDF) from the original on July 22, 2016. Retrieved December 19, 2016.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Yirka, Bob. "Researchers find carbon reactions with basalt can form carbonate minerals faster than thought". Phys.org. Omicron Technology Ltd. Archived from the original on April 26, 2014. Retrieved April 25, 2014.
- ^ a b Matter, Juerg M.; Stute, Martin; Snæbjörnsdottir, Sandra O.; Oelkers, Eric H.; Gislason, Sigurdur R.; Aradottir, Edda S.; Sigfusson, Bergur; Gunnarsson, Ingvi; Sigurdardottir, Holmfridur; Gunlaugsson, Einar; Axelsson, Gudni; Alfredsson, Helgi A.; Wolff-Boenisch, Domenik; Mesfin, Kiflom; Fernandez de la Reguera Taya, Diana; Hall, Jennifer; Dideriksen, Knud; Broecker, Wallace S. (June 10, 2016). "Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions". Science. 352 (6291): 1312–1314. Bibcode:2016Sci...352.1312M. doi:10.1126/science.aad8132. PMID 27284192.
- ^ Peter B. Kelemen1 and Jürg Matter (November 3, 2008). "In situ carbonation of peridotite for CO
2 storage". Proc. Natl. Acad. Sci. USA. 105 (45): 17295–300. Bibcode:2008PNAS..10517295K. doi:10.1073/pnas.0805794105. PMC 2582290.{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Timothy Gardner (November 7, 2008). "Scientists say a rock can soak up carbon dioxide | Reuters". Uk.reuters.com. Archived from the original on December 18, 2008. Retrieved May 9, 2010.
- ^ Le Page, Michael (June 19, 2016). "CO2 injected deep underground turns to rock – and stays there". New Scientist. Archived from the original on December 5, 2017. Retrieved December 4, 2017.
- ^ Proctor, Darrell (December 1, 2017). "Test of Carbon Capture Technology Underway at Iceland Geothermal Plant". POWER Magazine. Archived from the original on December 5, 2017. Retrieved December 4, 2017.
- ^ "This carbon-sucking mineral could help slow down climate change". Fast Company. 2018. Archived from the original on August 20, 2018. Retrieved August 20, 2018.
- ^ Ravikumar, Dwarakanath; Zhang, Duo; Keoleian, Gregory; Miller, Shelie; Sick, Volker; Li, Victor (February 8, 2021). "Carbon dioxide utilization in concrete curing or mixing might not produce a net climate benefit". Nature Communications. 12 (1): 855. Bibcode:2021NatCo..12..855R. doi:10.1038/s41467-021-21148-w. ISSN 2041-1723. PMC 7870952. PMID 33558537.
- ^ Andrew, Robbie M. (January 26, 2018). "Global CO2 emissions from cement production". Earth System Science Data. 10 (1): 195–217. Bibcode:2018ESSD...10..195A. doi:10.5194/essd-10-195-2018. ISSN 1866-3508.
- ^ Jorat, M.; Aziz, Maniruzzaman; Marto, Aminaton; Zaini, Nabilah; Jusoh, Siti; Manning, David (2018). "Sequestering Atmospheric CO2 Inorganically: A Solution for Malaysia's CO2 Emission". Geosciences. 8 (12): 483. Bibcode:2018Geosc...8..483J. doi:10.3390/geosciences8120483.
- ^ Skocek, Jan; Zajac, Maciej; Ben Haha, Mohsen (March 27, 2020). "Carbon Capture and Utilization by mineralization of cement pastes derived from recycled concrete". Scientific Reports. 10 (1): 5614. Bibcode:2020NatSR..10.5614S. doi:10.1038/s41598-020-62503-z. ISSN 2045-2322. PMC 7101415. PMID 32221348.
- ^ Zajac, Maciej; Skocek, Jan; Skibsted, Jørgen; Haha, Mohsen Ben (July 15, 2021). "CO2 mineralization of demolished concrete wastes into a supplementary cementitious material – a new CCU approach for the cement industry". RILEM Technical Letters. 6: 53–60. doi:10.21809/rilemtechlett.2021.141. ISSN 2518-0231. S2CID 237848467.
- ^ Esrafilzadeh, Dorna; Zavabeti, Ali; Jalili, Rouhollah; Atkin, Paul; Choi, Jaecheol; Carey, Benjamin J.; Brkljača, Robert; O’Mullane, Anthony P.; Dickey, Michael D.; Officer, David L.; MacFarlane, Douglas R.; Daeneke, Torben; Kalantar-Zadeh, Kourosh (February 26, 2019). "Room temperature CO 2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces". Nature Communications. 10 (1): 865. Bibcode:2019NatCo..10..865E. doi:10.1038/s41467-019-08824-8. PMC 6391491. PMID 30808867.
- ^ "Climate rewind: Scientists turn carbon dioxide back into coal". www.rmit.edu.au. Archived from the original on May 11, 2019. Retrieved May 11, 2019.
- ^ "Scientists turn CO2 'back into coal' in breakthrough carbon capture experiment". The Independent. February 26, 2019. Archived from the original on May 13, 2019. Retrieved May 11, 2019.
- ^ Irving, Michael (January 19, 2022). "Liquid metal catalyst quickly coverts carbon dioxide into solid carbon". New Atlas. Archived from the original on January 19, 2022. Retrieved January 19, 2022.
- ^ "Novacem". Imperial Innovations. May 6, 2008. Archived from the original on August 3, 2009. Retrieved May 9, 2010.
- ^ Jha, Alok (December 31, 2008). "Revealed: The cement that eats carbon dioxide". The Guardian. London. Archived from the original on August 6, 2013. Retrieved April 3, 2010.
- ^ "Home". TecEco. July 1, 1983. Archived from the original on April 27, 2010. Retrieved May 9, 2010.
- ^ Lord, Bronte. "This concrete can trap CO2 emissions forever". CNNMoney. Archived from the original on June 11, 2020. Retrieved June 17, 2018.
- ^ "UCLA researchers turn carbon dioxide into sustainable concrete". Archived from the original on December 17, 2018. Retrieved December 17, 2018.
- ^ Uibu, Mai; Uus, Mati; Kuusik, Rein (February 2008). "CO
2 mineral sequestration in oil-shale wastes from Estonian power production". Journal of Environmental Management. 90 (2): 1253–60. doi:10.1016/j.jenvman.2008.07.012. PMID 18793821. - ^ Chang, Kenneth (February 19, 2008). "Scientists Would Turn Greenhouse Gas Into Gasoline". The New York Times. Archived from the original on February 5, 2015. Retrieved April 3, 2010.
- ^ Frank Zeman (2007). "Energy and Material Balance of CO2 Capture from Ambient Air". Environ. Sci. Technol. 41 (21): 7558–63. Bibcode:2007EnST...41.7558Z. doi:10.1021/es070874m. PMID 18044541. S2CID 27280943.
- ^ "Chemical 'sponge' could filterCO
2 from the air". New Scientist. October 3, 2007. Archived from the original on February 26, 2010. Retrieved May 9, 2010. - ^ "New Device Vacuums Away Carbon Dioxide". LiveScience. May 1, 2007. Archived from the original on October 7, 2008. Retrieved May 9, 2010.
- ^ Adam, David (May 31, 2008). "Could US scientist's 'CO
2 catcher' help to slow warming?". The Guardian. London. Archived from the original on September 2, 2013. Retrieved April 3, 2010. - ^ Ortega, Alejandra; Geraldi, N.R.; Alam, I.; Kamau, A.A.; Acinas, S.; Logares, R.; Gasol, J.; Massana, R.; Krause-Jensen, D.; Duarte, C. (2019). "Important contribution of macroalgae to oceanic carbon sequestration". Nature Geoscience. 12 (9): 748–754. Bibcode:2019NatGe..12..748O. doi:10.1038/s41561-019-0421-8. hdl:10754/656768. S2CID 199448971. Archived from the original on May 6, 2021. Retrieved July 18, 2020.
- ^ Temple, James (September 19, 2021). "Companies hoping to grow carbon-sucking kelp may be rushing ahead of the science". MIT Technology Review. Archived from the original on September 19, 2021. Retrieved November 25, 2021.
- ^ Flannery, Tim (November 20, 2015). "Climate crisis: seaweed, coffee and cement could save the planet". The Guardian. Archived from the original on November 24, 2015. Retrieved November 25, 2015.
- ^ Vanegasa, C. H.; Bartletta, J. (February 11, 2013). "Green energy from marine algae: biogas production and composition from the anaerobic digestion of Irish seaweed species". Environmental Technology. 34 (15): 2277–2283. doi:10.1080/09593330.2013.765922. PMID 24350482. S2CID 30863033.
- ^ Matear, R. J. & B. Elliott (2004). "Enhancement of oceanic uptake of anthropogenic CO2 by macronutrient fertilization". J. Geophys. Res. 109 (C4): C04001. Bibcode:2004JGRC..109.4001M. doi:10.1029/2000JC000321. Archived from the original on March 4, 2010. Retrieved January 19, 2009.
- ^ Jones, I.S.F. & Young, H.E. (1997). "Engineering a large sustainable world fishery". Environmental Conservation. 24 (2): 99–104. doi:10.1017/S0376892997000167. S2CID 86248266.
- ^ Trujillo, Alan (2011). Essentials of Oceanography. Pearson Education, Inc. p. 157. ISBN 9780321668127.
- ^ a b National Academies of Sciences, Engineering (December 8, 2021). A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration. doi:10.17226/26278. ISBN 978-0-309-08761-2. PMID 35533244. S2CID 245089649.
- ^ "Cloud spraying and hurricane slaying: how ocean geoengineering became the frontier of the climate crisis". The Guardian. June 23, 2021. Archived from the original on June 23, 2021. Retrieved June 23, 2021.
- ^ a b Lovelock, James E.; Rapley, Chris G. (September 27, 2007). "Ocean pipes could help the earth to cure itself". Nature. 449 (7161): 403. Bibcode:2007Natur.449..403L. doi:10.1038/449403a. PMID 17898747.
- ^ Pearce, Fred (September 26, 2007). "Ocean pumps could counter global warming". New Scientist. Archived from the original on April 23, 2009. Retrieved May 9, 2010.
- ^ Duke, John H. (2008). "A proposal to force vertical mixing of the Pacific Equatorial Undercurrent to create a system of equatorially trapped coupled convection that counteracts global warming" (PDF). Geophysical Research Abstracts. Archived (PDF) from the original on July 13, 2011. Retrieved May 9, 2010.
- ^ Dutreuil, S.; Bopp, L.; Tagliabue, A. (May 25, 2009). "Impact of enhanced vertical mixing on marine biogeochemistry: lessons for geo-engineering and natural variability". Biogeosciences. 6 (5): 901–912. Bibcode:2009BGeo....6..901D. doi:10.5194/bg-6-901-2009. Archived from the original on September 23, 2015. Retrieved August 21, 2015.
- ^ "Ocean temperature". Science Learning Hub. Retrieved November 28, 2018.
- ^ Pearce, Fred. "Ocean pumps could counter global warming". New Scientist. Retrieved November 28, 2018.
- ^ Duke, John H. (2008). "A proposal to force vertical mixing of the Pacific Equatorial Undercurrent to create a system of equatorially trapped coupled convection that counteracts global warming" (PDF). Geophysical Research Abstracts.
- ^ US EPA, OW (June 3, 2013). "Harmful Algal Blooms | US EPA". US EPA. Retrieved November 28, 2018.
- ^ Shirley, Jolene S. "Discovering the Effects of Carbon Dioxide Levels on Marine Life and Global Climate". soundwaves.usgs.gov. Retrieved November 28, 2018.
- ^ David S. Goldberg; Taro Takahashi; Angela L. Slagle (2008). "Carbon dioxide sequestration in deep-sea basalt". Proc. Natl. Acad. Sci. USA. 105 (29): 9920–25. Bibcode:2008PNAS..105.9920G. doi:10.1073/pnas.0804397105. PMC 2464617. PMID 18626013.
- ^ a b "Carbon storage in undersea basalt offers extra security". environmentalresearchweb. July 15, 2008. Archived from the original on August 2, 2009. Retrieved May 9, 2010.
- ^ "Scientists turn carbon dioxide into stone to combat global warming". The Verge. Vox Media. June 10, 2016. Archived from the original on June 11, 2016. Retrieved June 11, 2016.
- ^ Goldthorpe, Steve (July 1, 2017). "Potential for Very Deep Ocean Storage of CO2 Without Ocean Acidification: A Discussion Paper". Energy Procedia. 114: 5417–5429. doi:10.1016/j.egypro.2017.03.1686. ISSN 1876-6102.
- ^ House, Kurt (November 10, 2005). "Permanent carbon dioxide storage in deep-sea sediments" (PDF). Proceedings of the National Academy of Sciences. 103 (33): 12291–12295. Bibcode:2006PNAS..10312291H. doi:10.1073/pnas.0605318103. PMC 1567873. PMID 16894174.
- ^ RIDGWELL, ANDY (January 13, 2007). "Regulation of atmospheric CO2 by deep-sea sediments in an Earth System Model" (PDF). Global Biogeochemical Cycles. 21 (2): GB2008. Bibcode:2007GBioC..21.2008R. doi:10.1029/2006GB002764. S2CID 55985323.
- ^ a b "Archived copy" (PDF). Archived from the original (PDF) on June 12, 2018. Retrieved December 8, 2018.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Qanbari, Farhad; Pooladi-Darvish, Mehran; Tabatabaie, S. Hamed; Gerami, Shahab (September 1, 2012). "CO2 disposal as hydrate in ocean sediments". Journal of Natural Gas Science and Engineering. 8: 139–149. doi:10.1016/j.jngse.2011.10.006. ISSN 1875-5100.
- ^ Zhang, Dongxiao; Teng, Yihua (July 1, 2018). "Long-term viability of carbon sequestration in deep-sea sediments". Science Advances. 4 (7): eaao6588. Bibcode:2018SciA....4O6588T. doi:10.1126/sciadv.aao6588. ISSN 2375-2548. PMC 6031374. PMID 29978037.
- ^ Kheshgi, H.S. (1995). "Sequestering atmospheric carbon dioxide by increasing ocean alkalinity". Energy. 20 (9): 915–922. doi:10.1016/0360-5442(95)00035-F.
- ^ K.S. Lackner; C.H. Wendt; D.P. Butt; E.L. Joyce; D.H. Sharp (1995). "Carbon dioxide disposal in carbonate minerals". Energy. 20 (11): 1153–70. doi:10.1016/0360-5442(95)00071-N.
- ^ K.S. Lackner; D.P. Butt; C.H. Wendt (1997). "Progress on binding CO
2 in mineral substrates". Energy Conversion and Management (Submitted manuscript). 38: S259–S264. doi:10.1016/S0196-8904(96)00279-8. Archived from the original on August 24, 2019. Retrieved July 31, 2018. - ^ Rau, Greg H.; Caldeira, Ken (November 1999). "Enhanced carbonate dissolution: A means of sequestering waste CO
2 as ocean bicarbonate". Energy Conversion and Management. 40 (17): 1803–1813. doi:10.1016/S0196-8904(99)00071-0. Archived from the original on June 10, 2020. Retrieved March 7, 2020. - ^ Rau, Greg H.; Knauss, Kevin G.; Langer, William H.; Caldeira, Ken (August 2007). "Reducing energy-related CO
2 emissions using accelerated weathering of limestone". Energy. 32 (8): 1471–7. doi:10.1016/j.energy.2006.10.011. - ^ Harvey, L.D.D. (2008). "Mitigating the atmospheric CO
2 increase and ocean acidification by adding limestone powder to upwelling regions". Journal of Geophysical Research. 113: C04028. Bibcode:2008JGRC..11304028H. doi:10.1029/2007JC004373. S2CID 54827652. - ^ "Scientists enhance Mother Nature's carbon handling mechanism". Penn State Live. November 7, 2007. Archived from the original on June 3, 2010.
- ^ Kurt Zenz House; Christopher H. House; Daniel P. Schrag; Michael J. Aziz (2007). "Electrochemical Acceleration of Chemical Weathering as an Energetically Feasible Approach to Mitigating Anthropogenic Climate Change". Environ. Sci. Technol. 41 (24): 8464–8470. Bibcode:2007EnST...41.8464H. doi:10.1021/es0701816. PMID 18200880.
- ^ Clover, Charles (November 7, 2007). "Global warming 'cure' found by scientists". The Daily Telegraph. London. Archived from the original on April 11, 2009. Retrieved April 3, 2010.
- ^ La Plante, Erika Callagon; Simonetti, Dante A.; Wang, Jingbo; Al-Turki, Abdulaziz; Chen, Xin; Jassby, David; Sant, Gaurav N. (January 25, 2021). "Saline Water-Based Mineralization Pathway for Gigatonne-Scale CO2 Management". ACS Sustainable Chemistry & Engineering. 9 (3): 1073–1089. doi:10.1021/acssuschemeng.0c08561. S2CID 234293936.
- ^ a b IPCC, 2005: IPCC Special Report on Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change [Metz, B., O. Davidson, H. C. de Coninck, M. Loos, and L. A. Meyer (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 442 pp.
- ^ IPCC, 2014: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Edenhofer, O., R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlömer, C. von Stechow, T. Zwickel and J.C. Minx (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- ^ IPCC (2022) Chapter 12: Cross sectoral perspectives Archived 13 October 2022 at the Wayback Machine in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Archived 2 August 2022 at the Wayback Machine, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
- ^ "Is carbon capture too expensive? – Analysis". IEA. Retrieved November 30, 2021.
- ^ "This startup has unlocked a novel way to capture carbon—by turning the dirty gas into rocks". Fortune. Retrieved December 1, 2021.
- ^ Austin, K. G.; Baker, J. S.; Sohngen, B. L.; Wade, C. M.; Daigneault, A.; Ohrel, S. B.; Ragnauth, S.; Bean, A. (December 1, 2020). "The economic costs of planting, preserving, and managing the world's forests to mitigate climate change". Nature Communications. 11 (1): 5946. Bibcode:2020NatCo..11.5946A. doi:10.1038/s41467-020-19578-z. ISSN 2041-1723. PMC 7708837. PMID 33262324.
- ^ Woodward, Aylin. "The world's biggest carbon-removal plant just opened. In a year, it'll negate just 3 seconds' worth of global emissions". Business Insider. Retrieved November 30, 2021.
- ^ Bjørn, Anders; Lloyd, Shannon M.; Brander, Matthew; Matthews, H. Damon (June 9, 2022). "Renewable energy certificates threaten the integrity of corporate science-based targets". Nature Climate Change. 12 (6): 539–546. Bibcode:2022NatCC..12..539B. doi:10.1038/s41558-022-01379-5. ISSN 1758-6798. S2CID 249524667.
- ^ Meredith, Sam (February 7, 2022). "World's biggest companies accused of exaggerating their climate actions". CNBC. Retrieved June 8, 2022.
- ^ "Battle over carbon capture as tool to fight climate change". AP NEWS. April 13, 2022. Retrieved June 8, 2022.
- ^ "Scientists urge end to fossil fuel use as landmark IPCC report readied". The Guardian. April 3, 2022. Retrieved June 11, 2022.
- ^ "Climate change: IPCC scientists say it's 'now or never' to limit warming". BBC News. April 4, 2022. Retrieved June 10, 2022.
- ^ Project, Stanford Solutions (May 21, 2022). "Why not Carbon Capture?". Medium. Retrieved June 8, 2022.
- ^ "Executive Order on Tackling the Climate Crisis at Home and Abroad". The White House. January 27, 2021. Archived from the original on February 17, 2021. Retrieved April 28, 2021.
External links
- Carbon Sequestration Leadership Forum International carbon capture and storage initiative.
- UK Carbon Capture and Storage Consortium Overview of the UK academic consortium focused on researching issues related to Carbon Capture and Storage.
- Carbon Capture & Sequestration Technologies MIT program covers carbon capture and storage (CCS)
