Solid-state chemistry: Difference between revisions
Added a sentence to the lead to reflect the presence of the synthetic methods section |
→Compositions and structures: added new content for XRD |
||
| Line 46: | Line 46: | ||
Once the unit cell of a new phase is known, the next step is to establish the stoichiometry of the phase. This can be done in several ways. Sometimes the composition of the original mixture will give a clue, under the circumstances that only a product with a single powder pattern is found or a phase of a certain composition is made by analogy to known material, but this is rare. |
Once the unit cell of a new phase is known, the next step is to establish the stoichiometry of the phase. This can be done in several ways. Sometimes the composition of the original mixture will give a clue, under the circumstances that only a product with a single powder pattern is found or a phase of a certain composition is made by analogy to known material, but this is rare. |
||
Often, considerable effort in refining the synthetic procedures is required to obtain a pure sample of the new material. If it is possible to separate the product from the rest of the reaction mixture, [[elemental analysis]] methods such as [[Scanning electron microscope|scanning electron microscopy]] (SEM) and [[transmission electron microscopy]] (TEM) can be used. The detection of scattered and transmitted electrons from the surface of the sample provides information about the surface topography and composition of the material.<ref>{{Cite book |url=http://link.springer.com/10.1007/978-3-319-92955-2 |title=Handbook of Materials Characterization |date=2018 |publisher=Springer International Publishing |isbn=978-3-319-92954-5 |editor-last=Sharma |editor-first=Surender Kumar |location=Cham |language=en |doi=10.1007/978-3-319-92955-2}}</ref> [[Energy-dispersive X-ray spectroscopy|Energy dispersive X-ray spectroscopy]] (EDX) is a technique that uses electron beam excitation. Exciting the inner shell of an atom with incident electrons emits characteristic X-rays with specific energy to each element.<ref name=": |
Often, considerable effort in refining the synthetic procedures is required to obtain a pure sample of the new material. If it is possible to separate the product from the rest of the reaction mixture, [[elemental analysis]] methods such as [[Scanning electron microscope|scanning electron microscopy]] (SEM) and [[transmission electron microscopy]] (TEM) can be used. The detection of scattered and transmitted electrons from the surface of the sample provides information about the surface topography and composition of the material.<ref name=":5">{{Cite book |url=http://link.springer.com/10.1007/978-3-319-92955-2 |title=Handbook of Materials Characterization |date=2018 |publisher=Springer International Publishing |isbn=978-3-319-92954-5 |editor-last=Sharma |editor-first=Surender Kumar |location=Cham |language=en |doi=10.1007/978-3-319-92955-2}}</ref> [[Energy-dispersive X-ray spectroscopy|Energy dispersive X-ray spectroscopy]] (EDX) is a technique that uses electron beam excitation. Exciting the inner shell of an atom with incident electrons emits characteristic X-rays with specific energy to each element.<ref name=":6">{{Cite book |last=Bell |first=Dc |url=https://www.taylorfrancis.com/books/9781135331405 |title=Energy Dispersive X-ray Analysis in the Electron Microscope |last2=Garratt-Reed |first2=Aj |date=2003-07-10 |publisher=Garland Science |isbn=978-1-135-33140-5 |edition=0 |language=en |doi=10.4324/9780203483428}}</ref> The peak energy can identify the chemical composition of a sample, including the distribution and concentration.<ref name=":6" />[[File:XRD_(Whole).jpg|thumb|An X-ray diffractometer (XRD) used to identify the crystalline phases in the material]]Similar to EDX, [[X-ray crystallography|X-ray diffraction]] analysis (XRD) involves the generation of characteristic X-rays upon interaction with the sample. The intensity of diffracted rays scattered at different angles is used to analyze the physical properties of a material such as phase composition and crystallographic structure.<ref>{{Cite book |last=Waseda |first=Yoshio |url=https://link.springer.com/10.1007/978-3-642-16635-8 |title=X-Ray Diffraction Crystallography: Introduction, Examples and Solved Problems |last2=Matsubara |first2=Eiichiro |last3=Shinoda |first3=Kozo |date=2011 |publisher=Springer Berlin Heidelberg |isbn=978-3-642-16634-1 |location=Berlin, Heidelberg |language=en |doi=10.1007/978-3-642-16635-8.}}</ref> These techniques can also be coupled to achieve a better effect. For example, SEM is a useful complement to EDX due to its focused electron beam, it produces a high-magnification image that provides information on the surface topography.<ref name=":5" /> Once the area of interest has been identified, EDX can be used to determine the elements present in that specific spot. [[Selected area diffraction|Selected area electron diffraction]] can be coupled with TEM or SEM to investigate the level of crystallinity and the lattice parameters of a sample.<ref>{{Cite journal |last=Zhou |first=Wuzong |last2=Greer |first2=Heather F. |date=2016-03 |title=What Can Electron Microscopy Tell Us Beyond Crystal Structures? |url=https://onlinelibrary.wiley.com/doi/10.1002/ejic.201501342 |journal=European Journal of Inorganic Chemistry |language=en |volume=2016 |issue=7 |pages=941–950 |doi=10.1002/ejic.201501342 |issn=1434-1948}}</ref> |
||
=== And more === |
|||
| ⚫ | X-ray diffraction is also used due to its imaging capabilities and speed of data generation.<ref>{{Cite journal|last=Schülli|first=Tobias U.|date=September 2018|title=X-ray nanobeam diffraction imaging of materials|journal=Current Opinion in Solid State and Materials Science|volume=22|issue=5|pages=188–201|doi=10.1016/j.cossms.2018.09.003|bibcode=2018COSSM..22..188S|doi-access=free}}</ref> The latter often requires ''revisiting'' and refining the preparative procedures and that are linked to the question of which phases are stable at what composition and what stoichiometry. In other words, what the [[phase diagram]] looks like.<ref>cf. Chapter 12 of Elements of X-ray diffraction, B.D. Cullity, Addison-Wesley, 2nd ed. 1977 {{ISBN|0-201-01174-3}}</ref> An important tool in establishing this are [[thermal analysis]] techniques like [[Differential scanning calorimetry|DSC]] or [[Differential thermal analysis|DTA]] and increasingly also, due to the advent of [[synchrotron]]s, temperature-dependent powder diffraction. Increased knowledge of the phase relations often leads to further |
||
| ⚫ | X-ray diffraction is also used due to its imaging capabilities and speed of data generation.<ref>{{Cite journal|last=Schülli|first=Tobias U.|date=September 2018|title=X-ray nanobeam diffraction imaging of materials|journal=Current Opinion in Solid State and Materials Science|volume=22|issue=5|pages=188–201|doi=10.1016/j.cossms.2018.09.003|bibcode=2018COSSM..22..188S|doi-access=free}}</ref> The latter often requires ''revisiting'' and refining the preparative procedures and that are linked to the question of which phases are stable at what composition and what stoichiometry. In other words, what the [[phase diagram]] looks like.<ref>cf. Chapter 12 of Elements of X-ray diffraction, B.D. Cullity, Addison-Wesley, 2nd ed. 1977 {{ISBN|0-201-01174-3}}</ref> An important tool in establishing this are [[thermal analysis]] techniques like [[Differential scanning calorimetry|DSC]] or [[Differential thermal analysis|DTA]] and increasingly also, due to the advent of [[synchrotron]]s, temperature-dependent powder diffraction. Increased knowledge of the phase relations often leads to further |
||
refinement in synthetic procedures in an iterative way. New phases are thus characterized by their melting points and their stoichiometric domains. The latter is important for the many solids that are non-stoichiometric compounds. The cell parameters obtained from XRD are particularly helpful to characterize the homogeneity ranges of the latter. |
refinement in synthetic procedures in an iterative way. New phases are thus characterized by their melting points and their stoichiometric domains. The latter is important for the many solids that are non-stoichiometric compounds. The cell parameters obtained from XRD are particularly helpful to characterize the homogeneity ranges of the latter. |
||
Revision as of 04:24, 16 April 2023
Solid-state chemistry, also sometimes referred as materials chemistry, is the study of the synthesis, structure, and properties of solid phase materials, particularly, but not necessarily exclusively of, non-molecular solids. It therefore has a strong overlap with solid-state physics, mineralogy, crystallography, ceramics, metallurgy, thermodynamics, materials science and electronics with a focus on the synthesis of novel materials and their characterisation. A diverse range of synthetic techniques, such as the ceramic method and chemical vapour depostion, make solid-state materials. Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles.[1]
History
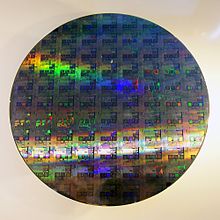
Because of its direct relevance to products of commerce, solid state inorganic chemistry has been strongly driven by technology. Progress in the field has often been fueled by the demands of industry, sometimes in collaboration with academia.[2] Applications discovered in the 20th century include zeolite and platinum-based catalysts for petroleum processing in the 1950s, high-purity silicon as a core component of microelectronic devices in the 1960s, and “high temperature” superconductivity in the 1980s. The invention of X-ray crystallography in the early 1900s by William Lawrence Bragg was an enabling innovation. Our understanding of how reactions proceed at the atomic level in the solid state was advanced considerably by Carl Wagner's work on oxidation rate theory, counter diffusion of ions, and defect chemistry. Because of his contributions, he has sometimes been referred to as the father of solid state chemistry.[3]
Synthetic methods
Given the diversity of solid-state compounds, an equally diverse array of methods are used for their preparation.[1][4] Synthesis can range from high-temperature methods, like the ceramic method, to gas methods, like chemical vapour deposition. Often, the methods prevent defect formation[5] or produce high-purity products[6].
High-temperature methods
Ceramic method
The ceramic method is one of the most common synthesis techniques.[7] The synthesis occurs entirely in the solid state.[7] The reactants are ground together, formed into a pellet, and heated at high temperatures in an oven.[7] After the precursors are reacted at a high temperature, the oven temperature must be gradually lowered to prevent defects and form a well-ordered crystal.[8]
Using a mortar and pestle or ball mill, the reactants are ground together, which decreases size and increases surface area of the reactants.[9] If the mixing is not sufficient, we can use techniques such as co-precipitation and sol-gel.[7] A chemist forms pellets from the ground reactants and places the pellets into containers for heating.[7] The choice of container depends on the precursors, the reaction temperature and the expected product.[7] For example, metal oxides are typically synthesized in silica or alumina containers.[7] A tube furnace heats the pellet.[7] Tube furnaces are available up to maximum temperatures of 2800oC.[10]

Melt methods
In the case of synthesizing glass ceramics, the synthetic technique involves melting together and then annealing the solidified melt.[11] The annealing temperature allows formation of crystalline structures within the glass.[11] When using volatile reactants, the reactants are put in an ampoule that is kept in liquid nitrogen. An oven heats the sealed ampoule. The solid can have abnormal grain growth (AGG), which may or may not be desirable.[12]
Low-temperature methods
Intercalation method
Intercalation synthesis is the insertion of molecules or ions between layers of a solid.[13] The layered solid has weak intermolecular bonds holding its layers together.[13] The process occurs via diffusion.[13] Intercalation is further driven by ion exchange, acid-base reactions or electrochemical reactions.[13] The intercalation method was first used in China with the discovery of porcelain. Also, graphene is produced by the intercalation method, and this method is the principle behind lithium-ion batteries.[14]
Solution methods
It is possible to use solvents to prepare solids by precipitation or by evaporation.[15] At times, the solvent is a hydrothermal that is under pressure at temperatures higher than the normal boiling point.[16] A variation on this theme is the use of flux methods, which use a salt with a relatively low melting point as the solvent.[17]
Gas methods
Many solids react vigorously with gas species like chlorine, iodine, and oxygen.[18][19] Other solids form adducts, such as CO or ethylene. Such reactions are conducted in open-ended tubes, which the gasses are passed through. Also, these reactions can take place inside a measuring device such as a TGA. In that case, stoichiometric information can be obtained during the reaction, which helps identify the products.
Chemical vapour transport
Chemical vapour transport results in very pure materials. The reaction typically occurs in a sealed ampoule.[20] A transporting agent, added to the sealed ampoule, produces a volatile intermediate species from the solid reactant.[20] For metal oxides, the transporting agent is usually Cl2 or HCl.[20] The ampoule has a temperature gradient, and, as the gaseous reactant travels along the gradient, it eventually deposits as a crystal.[20] An example of an industrially-used chemical vapor transport reaction is the Mond process. The Mond process involves heating impure nickel in a stream of carbon monoxide to produce pure nickel.[21]
Chemical vapour deposition
Chemical vapour deposition is a method widely used for the preparation of coatings and semiconductors from molecular precursors.[22] A carrier gas transports the gaseous precursors to the material for coating.[23]
Characterization
This is the process in which a material’s chemical composition, structure, and physical properties are determined using a variety of analytical techniques.
New phases
Synthetic methodology and characterization often go hand in hand in the sense that not one but a series of reaction mixtures are prepared and subjected to heat treatment. Stoichiometry, a numerical relationship between the quantities of reactant and product, is typically varied systematically. It is important to find which stoichiometries will lead to new solid compounds or solid solutions between known ones. A prime method to characterize the reaction products is powder diffraction because many solid-state reactions will produce polycrystalline molds or powders. Powder diffraction aids in the identification of known phases in the mixture.[24] If a pattern is found that is not known in the diffraction data libraries, an attempt can be made to index the pattern. The characterization of a material's properties is typically easier for a product with crystalline structures.
Compositions and structures

Once the unit cell of a new phase is known, the next step is to establish the stoichiometry of the phase. This can be done in several ways. Sometimes the composition of the original mixture will give a clue, under the circumstances that only a product with a single powder pattern is found or a phase of a certain composition is made by analogy to known material, but this is rare.
Often, considerable effort in refining the synthetic procedures is required to obtain a pure sample of the new material. If it is possible to separate the product from the rest of the reaction mixture, elemental analysis methods such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) can be used. The detection of scattered and transmitted electrons from the surface of the sample provides information about the surface topography and composition of the material.[25] Energy dispersive X-ray spectroscopy (EDX) is a technique that uses electron beam excitation. Exciting the inner shell of an atom with incident electrons emits characteristic X-rays with specific energy to each element.[26] The peak energy can identify the chemical composition of a sample, including the distribution and concentration.[26]

Similar to EDX, X-ray diffraction analysis (XRD) involves the generation of characteristic X-rays upon interaction with the sample. The intensity of diffracted rays scattered at different angles is used to analyze the physical properties of a material such as phase composition and crystallographic structure.[27] These techniques can also be coupled to achieve a better effect. For example, SEM is a useful complement to EDX due to its focused electron beam, it produces a high-magnification image that provides information on the surface topography.[25] Once the area of interest has been identified, EDX can be used to determine the elements present in that specific spot. Selected area electron diffraction can be coupled with TEM or SEM to investigate the level of crystallinity and the lattice parameters of a sample.[28]
And more
X-ray diffraction is also used due to its imaging capabilities and speed of data generation.[29] The latter often requires revisiting and refining the preparative procedures and that are linked to the question of which phases are stable at what composition and what stoichiometry. In other words, what the phase diagram looks like.[30] An important tool in establishing this are thermal analysis techniques like DSC or DTA and increasingly also, due to the advent of synchrotrons, temperature-dependent powder diffraction. Increased knowledge of the phase relations often leads to further
refinement in synthetic procedures in an iterative way. New phases are thus characterized by their melting points and their stoichiometric domains. The latter is important for the many solids that are non-stoichiometric compounds. The cell parameters obtained from XRD are particularly helpful to characterize the homogeneity ranges of the latter.
Local structure
In contrast to the large structures of crystals, the local structure describes the interaction of the nearest neighbouring atoms. Methods of nuclear spectroscopy use specific nuclei to probe the electric and magnetic fields around the nucleus. E.g. electric field gradients are very sensitive to small changes caused by lattice expansion/compression (thermal or pressure), phase changes, or local defects. Common methods are Mössbauer spectroscopy and perturbed angular correlation.
Further characterization
In many cases, new solid compounds are further characterized[31] by a variety of techniques that straddle the fine line that separates solid-state chemistry from solid-state physics. See Characterisation in material science for additional information.
Optical properties
For non-metallic materials, it is often possible to obtain UV/VIS spectra. In the case of semiconductors that will give an idea of the band gap.[32]
Citations
- ^ a b West, Anthony R. (2004). Solid State Chemistry and Its Applications. ISBN 981-253-003-7.
- ^ Kanatzidis, Mercouri G. (2018). "Report from the third workshop on future directions of solid-state chemistry: The status of solid-state chemistry and its impact in the physical sciences". Progress in Solid State Chemistry. 36 (1–2): 1–133. doi:10.1016/j.progsolidstchem.2007.02.002 – via Elsevier Science Direct.
- ^ Martin, Manfred (December 2002). "Life and achievements of Carl Wagner, 100th birthday". Solid State Ionics. 152–153: 15–17. doi:10.1016/S0167-2738(02)00318-1.
- ^ Cheetham, A. K.; Day, Peter (1988). Solid State Chemistry: Techniques. ISBN 0198552866.
- ^ Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.), "Synthesis Methods in Solid-State Chemistry", Synthesis Methods and Crystallization, IntechOpen, doi:10.5772/intechopen.93337, ISBN 978-1-83880-223-3, retrieved 2023-04-16
- ^ Mond, Ludwig; Langer, Carl; Quincke, Friedrich (1890-01-01). "L.—Action of carbon monoxide on nickel". Journal of the Chemical Society, Transactions. 57 (0): 749–753. doi:10.1039/CT8905700749. ISSN 0368-1645.
- ^ a b c d e f g h Rao, C. N. R. (2015). Essentials of inorganic materials synthesis. Kanishka Biswas. Hoboken, New Jersey. ISBN 1-118-89267-4. OCLC 908260711.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.), "Synthesis Methods in Solid-State Chemistry", Synthesis Methods and Crystallization, IntechOpen, doi:10.5772/intechopen.93337, ISBN 978-1-83880-223-3, retrieved 2023-04-16
- ^ Pagola, Silvina (2023-01). "Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View". Crystals. 13 (1): 124. doi:10.3390/cryst13010124. ISSN 2073-4352.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ "Tube Furnaces" (PDF). Retrieved March 30, 2023.
- ^ a b Antuzevics, Andris (2019-01-01), Shukla, Ashutosh Kumar (ed.), "Chapter 8 - EPR in glass ceramics", Experimental Methods in the Physical Sciences, Electron Magnetic Resonance - Applications in Physical Sciences and Biology, vol. 50, Academic Press, pp. 161–190, doi:10.1016/b978-0-12-814024-6.00008-x, retrieved 2023-04-16
- ^ Bednarczyk, Wiktor; Kawałko, Jakub; Rutkowski, Bogdan; Wątroba, Maria; Gao, Nong; Starink, Marco J.; Bała, Piotr; Langdon, Terence G. (2021-04-01). "Abnormal grain growth in a Zn-0.8Ag alloy after processing by high-pressure torsion". Acta Materialia. 207: 116667. doi:10.1016/j.actamat.2021.116667. ISSN 1359-6454.
- ^ a b c d Laipan, Minwang; Xiang, Lichen; Yu, Jingfang; Martin, Benjamin R.; Zhu, Runliang; Zhu, Jianxi; He, Hongping; Clearfield, Abraham; Sun, Luyi (2020-04-01). "Layered intercalation compounds: Mechanisms, new methodologies, and advanced applications". Progress in Materials Science. 109: 100631. doi:10.1016/j.pmatsci.2019.100631. ISSN 0079-6425.
- ^ Rajapakse, Manthila; Karki, Bhupendra; Abu, Usman O.; Pishgar, Sahar; Musa, Md Rajib Khan; Riyadh, S. M. Shah; Yu, Ming; Sumanasekera, Gamini; Jasinski, Jacek B. (2021-03-10). "Intercalation as a versatile tool for fabrication, property tuning, and phase transitions in 2D materials". npj 2D Materials and Applications. 5 (1): 1–21. doi:10.1038/s41699-021-00211-6. ISSN 2397-7132.
- ^ Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.), "Synthesis Methods in Solid-State Chemistry", Synthesis Methods and Crystallization, IntechOpen, doi:10.5772/intechopen.93337, ISBN 978-1-83880-223-3, retrieved 2023-04-16
- ^ Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.), "Synthesis Methods in Solid-State Chemistry", Synthesis Methods and Crystallization, IntechOpen, doi:10.5772/intechopen.93337, ISBN 978-1-83880-223-3, retrieved 2023-04-16
- ^ Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.), "Synthesis Methods in Solid-State Chemistry", Synthesis Methods and Crystallization, IntechOpen, doi:10.5772/intechopen.93337, ISBN 978-1-83880-223-3, retrieved 2023-04-16
- ^ Fromhold, Albert T.; Fromhold, Regina G. (1984-01-01), Bamford, C. H.; Tipper, C. F. H.; Compton, R. G. (eds.), "Chapter 1 An Overview of Metal Oxidation Theory", Comprehensive Chemical Kinetics, Reactions of Solids with Gases, vol. 21, Elsevier, pp. 1–117, doi:10.1016/s0069-8040(08)70006-2, retrieved 2023-04-03
- ^ Koga, Y.; Harrison, L. G. (1984-01-01), Bamford, C. H.; Tipper, C. F. H.; Compton, R. G. (eds.), "Chapter 2 Reactions of Solids with Gases other than Oxygen", Comprehensive Chemical Kinetics, Reactions of Solids with Gases, vol. 21, Elsevier, pp. 119–149, doi:10.1016/s0069-8040(08)70007-4, retrieved 2023-04-03
- ^ a b c d Binnewies, Michael; Glaum, Robert; Schmidt, Marcus; Schmidt, Peer (2013-02). "Chemical Vapor Transport Reactions - A Historical Review". Zeitschrift für anorganische und allgemeine Chemie. 639 (2): 219–229. doi:10.1002/zaac.201300048.
{{cite journal}}: Check date values in:|date=(help) - ^ Mond, Ludwig; Langer, Carl; Quincke, Friedrich (1890-01-01). "L.—Action of carbon monoxide on nickel". Journal of the Chemical Society, Transactions. 57 (0): 749–753. doi:10.1039/CT8905700749. ISSN 0368-1645.
- ^ Handbook of deposition technologies for films and coatings : science, applications and technology. Peter M. Martin (3rd ed ed.). Amsterdam: Elsevier. 2010. ISBN 978-0-08-095194-2. OCLC 670438909.
{{cite book}}:|edition=has extra text (help)CS1 maint: others (link) - ^ Vernardou, Dimitra (2020-01). "Special Issue: Advances in Chemical Vapor Deposition". Materials. 13 (18): 4167. doi:10.3390/ma13184167. ISSN 1996-1944. PMC 7560419. PMID 32961715.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Holder, Cameron F.; Schaak, Raymond E. (2019-07-23). "Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials". ACS Nano. 13 (7): 7359–7365. doi:10.1021/acsnano.9b05157. ISSN 1936-0851.
- ^ a b Sharma, Surender Kumar, ed. (2018). Handbook of Materials Characterization. Cham: Springer International Publishing. doi:10.1007/978-3-319-92955-2. ISBN 978-3-319-92954-5.
- ^ a b Bell, Dc; Garratt-Reed, Aj (2003-07-10). Energy Dispersive X-ray Analysis in the Electron Microscope (0 ed.). Garland Science. doi:10.4324/9780203483428. ISBN 978-1-135-33140-5.
- ^ Waseda, Yoshio; Matsubara, Eiichiro; Shinoda, Kozo (2011). X-Ray Diffraction Crystallography: Introduction, Examples and Solved Problems. Berlin, Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-16635-8.. ISBN 978-3-642-16634-1.
{{cite book}}: Check|doi=value (help) - ^ Zhou, Wuzong; Greer, Heather F. (2016-03). "What Can Electron Microscopy Tell Us Beyond Crystal Structures?". European Journal of Inorganic Chemistry. 2016 (7): 941–950. doi:10.1002/ejic.201501342. ISSN 1434-1948.
{{cite journal}}: Check date values in:|date=(help) - ^ Schülli, Tobias U. (September 2018). "X-ray nanobeam diffraction imaging of materials". Current Opinion in Solid State and Materials Science. 22 (5): 188–201. Bibcode:2018COSSM..22..188S. doi:10.1016/j.cossms.2018.09.003.
- ^ cf. Chapter 12 of Elements of X-ray diffraction, B.D. Cullity, Addison-Wesley, 2nd ed. 1977 ISBN 0-201-01174-3
- ^ cf. Chapter 2 of New directions in Solid State Chemistry. C. N. R. Rao and J. Gopalakrishnan. Cambridge U. Press 1997 ISBN 0-521-49559-8
- ^ Cox, P. A. (1995). Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties. Oxford Univ. Press. ISBN 978-0-19-958894-7.
External links
 Media related to Solid state chemistry at Wikimedia Commons
Media related to Solid state chemistry at Wikimedia Commons- [1], Sadoway, Donald. 3.091SC; Introduction to Solid State Chemistry, Fall 2010. (Massachusetts Institute of Technology: MIT OpenCourseWare)
