Intron: Difference between revisions
m →Introduction: syntax |
Narayanese (talk | contribs) →Introduction: replace |
||
| Line 13: | Line 13: | ||
Introns are common in [[eukaryote|eukaryotic]] pre-mRNA, but in [[prokaryote]]s they are only found in [[Transfer RNA|tRNA]] and [[Ribosomal RNA|rRNA]]. Introns, which are non-coding sections of a gene that are removed, are the opposite of [[exon]]s which remain in the mRNA sequence after processing. |
Introns are common in [[eukaryote|eukaryotic]] pre-mRNA, but in [[prokaryote]]s they are only found in [[Transfer RNA|tRNA]] and [[Ribosomal RNA|rRNA]]. Introns, which are non-coding sections of a gene that are removed, are the opposite of [[exon]]s which remain in the mRNA sequence after processing. |
||
The number and length of introns varies widely among [[species]], and among genes within the same species. |
The number and length of introns varies widely among [[species]], and among genes within the same species. Some eukaryotes, e.g. fungi and [[excavate]]s, have evolved streamlined genomes with few introns,<ref>{{cite journal |author=Stajich JE, Dietrich FS, Roy SW |title=Comparative genomic analysis of fungal genomes reveals intron-rich ancestors |journal=Genome Biol. |volume=8 |issue=10 |pages=R223 |year=2007 |pmid=17949488 |pmc=2246297 |doi=10.1186/gb-2007-8-10-r223}}</ref><ref>{{cite journal |author=Slamovits CH, Keeling PJ |title=A high density of ancient spliceosomal introns in oxymonad excavates |journal=BMC Evol. Biol. |volume=6 |issue= |pages=34 |year=2006 |pmid=16638131 |pmc=1501061 |doi=10.1186/1471-2148-6-34}}</ref> while the genomes of many other eukaryote groups are rich in introns.<ref>{{cite journal |author=Csurös M, Rogozin IB, Koonin EV |title=Extremely intron-rich genes in the alveolate ancestors inferred with a flexible maximum-likelihood approach |journal=Mol. Biol. Evol. |volume=25 |issue=5 |pages=903–11 |year=2008 |month=May |pmid=18296415 |doi=10.1093/molbev/msn039 |url=}}</ref><ref>{{cite journal |author=Smith DR, Lee RW |title=Nucleotide diversity in the mitochondrial and nuclear compartments of Chlamydomonas reinhardtii: investigating the origins of genome architecture |journal=BMC Evol. Biol. |volume=8 |issue= |pages=156 |year=2008 |pmid=18495022 |pmc=2412866 |doi=10.1186/1471-2148-8-156}}</ref> In humans, the gene with the greatest number of introns is the gene for the protein [[Titin]], with 362 introns.<ref>{{cite journal |author=Lander ES, Linton LM, Birren B, ''et al'' |title=Initial sequencing and analysis of the human genome |journal=Nature |volume=409 |issue=6822 |pages=860–921 |year=2001 |pmid=11237011 |doi=10.1038/35057062| url=http://www.euchromatin.org/Collins1.htm}}</ref> |
||
[[Image:Pre-mRNA to mRNA.png|right|thumbnail|420px|Simple illustration of a pre-mRNA, with introns (top). After the introns have been removed via splicing, the mature mRNA sequence is ready for translation (bottom).]] |
[[Image:Pre-mRNA to mRNA.png|right|thumbnail|420px|Simple illustration of a pre-mRNA, with introns (top). After the introns have been removed via splicing, the mature mRNA sequence is ready for translation (bottom).]] |
||
Revision as of 22:49, 11 August 2008
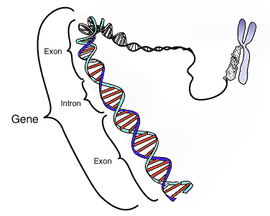
Introns, derived from the term "intragenic regions" and also called intervening sequence (IVS)[1], are non-coding sections of precursor mRNA (pre-mRNA) or other RNAs, that are removed (spliced out of the RNA) before the mature RNA is formed. Once the introns have been spliced out of a pre-mRNA, the resulting mRNA sequence, composed of exons, is ready to be translated into a protein. The corresponding parts of a gene are known as introns as well.
Introduction
Introns are common in eukaryotic pre-mRNA, but in prokaryotes they are only found in tRNA and rRNA. Introns, which are non-coding sections of a gene that are removed, are the opposite of exons which remain in the mRNA sequence after processing.
The number and length of introns varies widely among species, and among genes within the same species. Some eukaryotes, e.g. fungi and excavates, have evolved streamlined genomes with few introns,[2][3] while the genomes of many other eukaryote groups are rich in introns.[4][5] In humans, the gene with the greatest number of introns is the gene for the protein Titin, with 362 introns.[6]

Introns sometimes allow for alternative splicing of a gene, so that several different proteins which share some sequences in common can be translated from a single gene. The control of mRNA splicing is performed by a wide variety of signaling molecules.
Introns may also contain "old code", or sections of a gene that were once translated into a protein, but have since been discarded. It was generally assumed that the sequence of any given intron is junk DNA with no function. More recently, however, this is being disputed.[7]
Introns contain several short sequences that are important for efficient splicing. The exact mechanism for these intronic splicing enhancers is not well understood, but it is thought that they serve as binding sites on the transcript for proteins which stabilize the spliceosome. It is also possible that RNA secondary structure formed by intronic sequences may have an effect on splicing.
Discovery
The discovery of introns led to the Nobel Prize in Physiology or Medicine in 1993 for Phillip Allen Sharp and Richard J. Roberts. The term intron was introduced by American biochemist Walter Gilbert:[8]
"The notion of the cistron [...] must be replaced by that of a transcription unit containing regions which will be lost from the mature messenger - which I suggest we call introns (for intragenic regions) - alternating with regions which will be expressed - exons." (Gilbert 1978)
Classification of introns
Some introns, such as Group I and Group II introns, are actually ribozymes that are capable of catalyzing their own splicing out of a primary RNA transcript. This self splicing activity was discovered by Thomas Cech, who shared the 1989 Nobel Prize in Chemistry with Sidney Altman for the discovery of the catalytic properties of RNA.
Four classes of introns are known to exist:
Sometimes group III introns are also identified as group II introns, because of their similarity in structure and function.
Nuclear or spliceosomal introns are spliced by the spliceosome and a series of snRNAs (small nuclear RNAs). There are certain splice signals (or consensus sequences) which abet the splicing (or identification) of these introns by the spliceosome.
Group I, II and III introns are self splicing introns and are relatively rare compared to spliceosomal introns. Group II and III introns are similar and have a conserved secondary structure. The lariat pathway is used in their splicing. They perform functions similar to the spliceosome and may be evolutionarily related to it. Group I introns are the only class of introns whose splicing requires a free guanine nucleoside. They possess a secondary structure different from that of group II and III introns. Many self-splicing introns code for maturases that help with the splicing process, generally only the splicing of the intron that encodes it.[9]
Intron evolution
There are two competing theories that offer alternative scenarios for the origin and early evolution of spliceosomal introns (Other classes of introns such as self-splicing and tRNA introns are not subject to much debate, but see [10] for the former). These are popularly called as the Introns-Early (IE) or the Introns-Late (IL) views.[11]
The IE model, championed by Walter Gilbert,[12] proposes that introns are extremely old and numerously present in the earliest ancestors of prokaryotes and eukaryotes (the progenote). In this model introns were subsequently lost from prokaryotic organisms, allowing them to attain growth efficiency. A central prediction of this theory is that the early introns were mediators that facilitated the recombination of exons that represented the protein domains.[13] Such a model would directly lead to the evolution of new genes. Unfortunately, the model cannot account for the variations in the positions of shared introns between different species.[14]
The IL model proposes that introns were more recently inserted into original intron-less contiguous genes after the divergence of eukaryotes and prokaryotes. In this model, introns probably had their origin in parasitic transposable elements. This model is based on the observation that the spliceosomal introns are restricted to eukaryotes alone. However, there is considerable debate on the presence of introns in the early prokaryote-eukaryote ancestors and the subsequent intron loss-gain during eukaryotic evolution.[15] It is also suggested that the evolution of introns and more generally the intron-exon structure is largely independent of the coding-sequence evolution.[16]
Identification
Nearly all eukaryotic nuclear introns begin with the nucleotide sequence GU, and end with AG (the GU-AG rule). These, along with a larger consensus sequence, help direct the splicing machinery to the proper intronic donor and acceptor sites. This mainly occurs in eukaryotic primary mRNA transcripts.
See also
Structure:
Splicing:
Others:
References
- ^ Mark Lefers. "intron (intervening sequence)". Department of Biochemistry, Molecular Biology, and Cell Biology, Weinberg College of Arts & Sciences, Northwestern University. Retrieved 2008-06-17.
- ^ Stajich JE, Dietrich FS, Roy SW (2007). "Comparative genomic analysis of fungal genomes reveals intron-rich ancestors". Genome Biol. 8 (10): R223. doi:10.1186/gb-2007-8-10-r223. PMC 2246297. PMID 17949488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Slamovits CH, Keeling PJ (2006). "A high density of ancient spliceosomal introns in oxymonad excavates". BMC Evol. Biol. 6: 34. doi:10.1186/1471-2148-6-34. PMC 1501061. PMID 16638131.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Csurös M, Rogozin IB, Koonin EV (2008). "Extremely intron-rich genes in the alveolate ancestors inferred with a flexible maximum-likelihood approach". Mol. Biol. Evol. 25 (5): 903–11. doi:10.1093/molbev/msn039. PMID 18296415.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Smith DR, Lee RW (2008). "Nucleotide diversity in the mitochondrial and nuclear compartments of Chlamydomonas reinhardtii: investigating the origins of genome architecture". BMC Evol. Biol. 8: 156. doi:10.1186/1471-2148-8-156. PMC 2412866. PMID 18495022.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lander ES, Linton LM, Birren B; et al. (2001). "Initial sequencing and analysis of the human genome". Nature. 409 (6822): 860–921. doi:10.1038/35057062. PMID 11237011.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Crosio C, Cecconi F, Mariottini P, Cesareni G, Brenner S, Amaldi F (1996). "Fugu intron oversize reveals the presence of U15 snoRNA coding sequences in some introns of the ribosomal protein S3 gene". Genome Res. 6 (12): e15. doi:10.1101/gr.6.12.1227. PMID 8973918.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gilbert, Walter (1978). "Why genes in pieces". Nature. 271 (5645): 501. doi:10.1038/271501a0.
- ^ Doetsch NA, Thompson MD, Hallick RB (1998). "A maturase-encoding group III twintron is conserved in deeply rooted euglenoid species: are group III introns the chicken or the egg?". Mol. Biol. Evol. 15 (1): 76–86. PMID 9491607.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gogarten JP, Hilario E. "Inteins, introns, and homing endonucleases: recent revelations about the life cycle of parasitic genetic elements." BMC Evol Biol. 2006 Nov 13; 6: 94. PMID 17101053
- ^ Roy SW, Gilbert W (2006). "The evolution of spliceosomal introns: patterns, puzzles and progress" (PDF). Nat. Rev. Genet. 7 (3): 211–21. doi:10.1038/nrg1807. PMID 16485020.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Fedorov A, Cao X, Saxonov S, de Souza SJ, Roy SW, Gilbert W. "Intron distribution difference for 276 ancient and 131 modern genes suggests the existence of ancient introns." Proc Natl Acad Sci U S A. 2001 Nov 6; 98(23): 13177-82. PMID 11687643.
- ^ Rodriguez-Trelles F, Tarrio R, Ayala, FJ. "Origins and evolution of spliceosomal introns." Annual Review of Genetics 2006; 40: 47-76.
- ^ Rzhetsky A, Ayala FJ. "The enigma of intron origins." Cellular and Molecular Life Sciences 1999; 55(1): 3-6.
- ^ Sverdlov AV, Csuros M, Rogozin IB, Koonin EV. "A glimpse of a putative pre-intron phase of eukaryotic evolution." Trends Genet. 2007 Mar; 23(3): 105-108. PMID 17239982
- ^ Yandell M, Mungall CJ, Smith C; et al. (2006). "Large-scale trends in the evolution of gene structures within 11 animal genomes". PLoS Comput. Biol. 2 (3): e15. doi:10.1371/journal.pcbi.0020015. PMID 16518452.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
External links
