Epitestosterone: Difference between revisions
m Delink dates (WP:MOSUNLINKDATES) using Project:AWB |
m →Cite journal with Wikipedia template filling |
||
| Line 21: | Line 21: | ||
| specific_rotation= |
| specific_rotation= |
||
| sec_combustion=}} |
| sec_combustion=}} |
||
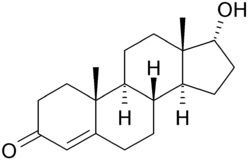
'''Epitestosterone''' is a natural [[steroid]], an inactive [[epimer]] of the hormone [[testosterone]]. Structurally, it differs from testosterone only in the configuration at the OH-bearing carbon, [[:File:Steroid numbering.png|C17]]. Epitestosterone is believed to form in a similar way to testosterone; a 1993 study found that around 50% of epitestosterone production in human males can be ascribed to the testis,<ref name= |
'''Epitestosterone''' is a natural [[steroid]], an inactive [[epimer]] of the hormone [[testosterone]]. Structurally, it differs from testosterone only in the configuration at the OH-bearing carbon, [[:File:Steroid numbering.png|C17]]. Epitestosterone is believed to form in a similar way to testosterone; a 1993 study found that around 50% of epitestosterone production in human males can be ascribed to the testis,<ref name=Dehennin93>{{cite journal |author=Dehennin L |title=Secretion by the human testis of epitestosterone, with its sulfoconjugate and precursor androgen 5-androstene-3 beta,17 α-diol |journal=J. Steroid Biochem. Mol. Biol. |volume=44 |issue=2 |pages=171–7 |year=1993 |month=February |pmid=8439521 }}</ref> although the exact pathway of its formation is still the subject of research. It has been shown to accumulate in mammary cyst fluid and in the [[prostate]].<ref name=Dehennin93/> Epitestosterone levels are typically highest in young males; however, by adulthood, most healthy males exhibit a testosterone to epitestosterone ratio (T/E ratio) of about 1:1.<ref>{{cite journal |author=Bellemare V, Faucher F, Breton R, Luu-The V |title=Characterization of 17α-hydroxysteroid dehydrogenase activity (17α-HSD) and its involvement in the biosynthesis of epitestosterone |journal=BMC Biochem. |volume=6 |issue= |pages=12 |year=2005 |pmid=16018803 |pmc=1185520 |doi=10.1186/1471-2091-6-12 |url=http://www.biomedcentral.com/1471-2091/6/12}}</ref> |
||
==Epitestosterone and testosterone== |
==Epitestosterone and testosterone== |
||
{{Disputed-section|date=January 2009}} |
{{Disputed-section|date=January 2009}} |
||
{{Weasel|date=November 2009}} |
{{Weasel|date=November 2009}} |
||
It has been shown that [[exogenous]] administration of [[testosterone]] does not affect levels of epitestosterone in the body. As a result, tests to determine the ratio of testosterone to epitestosterone in [[urine]] are used to find athletes who are [[Doping (Sport)|doping]].<ref> |
It has been shown that [[exogenous]] administration of [[testosterone]] does not affect levels of epitestosterone in the body. As a result, tests to determine the ratio of testosterone to epitestosterone in [[urine]] are used to find athletes who are [[Doping (Sport)|doping]].<ref>{{cite journal |author=Aguilera R, Hatton CK, Catlin DH |title=Detection of epitestosterone doping by isotope ratio mass spectrometry |journal=Clin. Chem. |volume=48 |issue=4 |pages=629–36 |year=2002 |pmid=11901061 |url=http://www.clinchem.org/cgi/pmidlookup?view=long&pmid=11901061}}</ref> Most persons have a ratio of about 1:1 testosterone to epitestosterone (T/E ratio) in their urine. However, it is not uncommon to find T/E ratios of up to 4:1 and even T/E ratios of 10:1 can be normal for some individuals. T/E tests are most common because a person may naturally have high levels of testosterone, but even so average T/E ratios for the population in general tends close to 1:1. |
||
Epitestosterone has not been shown to enhance athletic performance, although administration of epistestosterone can be used to mask a high level of testosterone if the standard T/E ratio test is used. As such, epitestosterone is banned by many sporting authorities as a masking agent for testosterone. |
Epitestosterone has not been shown to enhance athletic performance, although administration of epistestosterone can be used to mask a high level of testosterone if the standard T/E ratio test is used. As such, epitestosterone is banned by many sporting authorities as a masking agent for testosterone. |
||
| Line 39: | Line 39: | ||
== External links == |
== External links == |
||
* [http://news.bbc.co.uk/sport2/hi/other_sports/cycling/5237990.stm Landis has T/E ratio twice the tour limit] |
* [http://news.bbc.co.uk/sport2/hi/other_sports/cycling/5237990.stm Landis has T/E ratio twice the tour limit] |
||
*{{cite journal |author=Stárka L |title=Epitestosterone |journal=J. Steroid Biochem. Mol. Biol. |volume=87 |issue=1 |pages=27–34 |year=2003 |month=October |pmid=14630088 |url=http://linkinghub.elsevier.com/retrieve/pii/S0960076003003832}} |
|||
* [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14630088&dopt=Abstract Institute of Endocrinology Abstract on Epistestosterone] |
|||
*{{cite journal |author=Sanders BK |title=Sex, drugs and sports: prostaglandins, epitestosterone and sexual development |journal=Med. Hypotheses |volume=69 |issue=4 |pages=829–35 |year=2007 |pmid=17382481 |doi=10.1016/j.mehy.2006.12.058 |url=http://linkinghub.elsevier.com/retrieve/pii/S0306-9877(07)00150-8}} |
|||
* [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17382481 Sex, drugs and sports: Prostaglandins, epitestosterone and sexual development] |
|||
*{{cite journal |author=Loraine JA, Ismail AA, Adamopoulos DA, Dove GA |title=Endocrine function in male and female homosexuals |journal=Br Med J |volume=4 |issue=5732 |pages=406–9 |year=1970 |month=November |pmid=5481520 |pmc=1819981 }} |
|||
*[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1819981 Endocrine Functions in Male and Female Homosexuals] |
|||
*{{cite journal |author=Griffiths PD, Merry J, Browning MC, ''et al.'' |title=Homosexual women: an endocrine and psychological study |journal=J. Endocrinol. |volume=63 |issue=3 |pages=549–56 |year=1974 |month=December |pmid=4452820 |url=http://joe.endocrinology-journals.org/cgi/pmidlookup?view=long&pmid=4452820}} |
|||
*[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=4452820&query_hl=25&itool=pubmed_docsum Homosexual women: an endocrine and psychological study.] |
|||
{{Steroid metabolism intermediates}} |
{{Steroid metabolism intermediates}} |
||
Revision as of 13:31, 23 December 2009
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.169.813 |
| Chemical and physical data | |
| Formula | C19H28O2 |
| Molar mass | 288.42 g·mol−1 |
Epitestosterone is a natural steroid, an inactive epimer of the hormone testosterone. Structurally, it differs from testosterone only in the configuration at the OH-bearing carbon, C17. Epitestosterone is believed to form in a similar way to testosterone; a 1993 study found that around 50% of epitestosterone production in human males can be ascribed to the testis,[1] although the exact pathway of its formation is still the subject of research. It has been shown to accumulate in mammary cyst fluid and in the prostate.[1] Epitestosterone levels are typically highest in young males; however, by adulthood, most healthy males exhibit a testosterone to epitestosterone ratio (T/E ratio) of about 1:1.[2]
Epitestosterone and testosterone
This section's factual accuracy is disputed. (January 2009) |
This article contains weasel words: vague phrasing that often accompanies biased or unverifiable information. (November 2009) |
It has been shown that exogenous administration of testosterone does not affect levels of epitestosterone in the body. As a result, tests to determine the ratio of testosterone to epitestosterone in urine are used to find athletes who are doping.[3] Most persons have a ratio of about 1:1 testosterone to epitestosterone (T/E ratio) in their urine. However, it is not uncommon to find T/E ratios of up to 4:1 and even T/E ratios of 10:1 can be normal for some individuals. T/E tests are most common because a person may naturally have high levels of testosterone, but even so average T/E ratios for the population in general tends close to 1:1.
Epitestosterone has not been shown to enhance athletic performance, although administration of epistestosterone can be used to mask a high level of testosterone if the standard T/E ratio test is used. As such, epitestosterone is banned by many sporting authorities as a masking agent for testosterone.
In 1996 the US athlete Mary Decker failed a T/E test with a T/E ratio of greater than 6, the limit in force at the time. She took the case to arbitration, arguing that birth control pills can cause false positives for the test, but the arbitration panel ruled against her.
On September 20, 2007 Floyd Landis was stripped of his title as winner of the Tour de France, and was subjected to a two year ban from professional racing after a second test showing an elevated T/E ratio. Test results from Floyd Landis' "A" test sample indicated that while the ratio was 11:1, his testosterone level was in the normal range and the problem was actually a deficient level of epitestosterone[4]. Landis won the 17th stage of the tour; however, tests taken immediately after the stage victory showed a T/E ratio of 11:1[5], more than double the 4:1 imposed limit (recently lowered from prior limits of 8:1 and 6:1). On August 1, 2006, media reports said that synthetic testosterone had been detected in the A sample, using the carbon isotope ratio test CIR. The presence of synthetic testosterone means that some of the testosterone in Landis’s body came from an external source and was not naturally produced by his own system. These results conflict with Landis's public speculation that it was a natural occurrence.[6] Landis had emphatically denied the charge, pointing out the scientific data that testosterone cannot enhance athletic performance unless taken over an extended period of time with regular doses.
Notes
- ^ a b Dehennin L (1993). "Secretion by the human testis of epitestosterone, with its sulfoconjugate and precursor androgen 5-androstene-3 beta,17 α-diol". J. Steroid Biochem. Mol. Biol. 44 (2): 171–7. PMID 8439521.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bellemare V, Faucher F, Breton R, Luu-The V (2005). "Characterization of 17α-hydroxysteroid dehydrogenase activity (17α-HSD) and its involvement in the biosynthesis of epitestosterone". BMC Biochem. 6: 12. doi:10.1186/1471-2091-6-12. PMC 1185520. PMID 16018803.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Aguilera R, Hatton CK, Catlin DH (2002). "Detection of epitestosterone doping by isotope ratio mass spectrometry". Clin. Chem. 48 (4): 629–36. PMID 11901061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ This is unsubstantiated. Reference is needed
- ^ This is disputed. According to testimony offered in the USADA hearing, multiple test of the same sample produced results ranging from 4:1 up to 11:1. The lab chose to report the 11:1 figure.
- ^ "Synthetic testosterone found in Landis urine sample". Associated Press. 2006-07-31. Retrieved 2007-09-25.
External links
- Landis has T/E ratio twice the tour limit
- Stárka L (2003). "Epitestosterone". J. Steroid Biochem. Mol. Biol. 87 (1): 27–34. PMID 14630088.
{{cite journal}}: Unknown parameter|month=ignored (help) - Sanders BK (2007). "Sex, drugs and sports: prostaglandins, epitestosterone and sexual development". Med. Hypotheses. 69 (4): 829–35. doi:10.1016/j.mehy.2006.12.058. PMID 17382481.
- Loraine JA, Ismail AA, Adamopoulos DA, Dove GA (1970). "Endocrine function in male and female homosexuals". Br Med J. 4 (5732): 406–9. PMC 1819981. PMID 5481520.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Griffiths PD, Merry J, Browning MC; et al. (1974). "Homosexual women: an endocrine and psychological study". J. Endocrinol. 63 (3): 549–56. PMID 4452820.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
