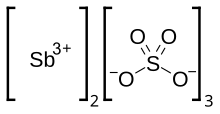
Antimony(III) sulfate
| |
| Names | |
|---|---|
| IUPAC name
Antimony(III) sulfate
| |
| Other names
Antimonous sulfate
Antimony trisulfate Diantimony trisulfate Diantimony tris(sulphate) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.370 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[2] | |
| Sb2(SO4)3 | |
| Molar mass | 531.7078 g/mol |
| Density | 3.94 g/cm3[1] |
| Hydrolysis[1] | |
| Structure[1] | |
| monoclinic | |
| P21/c | |
a = 13.12 Å, b = 4.75 Å, c = 17.55 Å α = 90°, β = 126.3°, γ = 90°
| |
Lattice volume (V)
|
881 Å3 |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[3] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[3] |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.[4]
Structure[edit]
Antimony(III) sulfate consists of interconnected SbO6 octahedra, which the corners are bonded to the sulfate ion.[1]
Production[edit]
Antimony(III) sulfate was first produced in 1827 by the reaction of antimony(III) oxide and 18 molar sulfuric acid at 200 °C:[1]
- Sb2O3 + 3 H2SO4 → Sb2(SO4)3 + 3 H2O
The concentration of the sulfuric acid is important, as a lower concentration will produce basic antimony oxides, while a higher concentration will produce antimony(III) pyrosulfate. The reaction of elemental antimony and 18 M sulfuric acid will also produce antimony(III) sulfate:[4]
- 2 Sb + 6 H2SO4 → Sb2(SO4)3 + 3 SO2 + 6 H2O
Chemical properties[edit]
Antimony sulfate is deliquescent, hydrolyzing in moist air and water, producing various basic antimony oxides and antimony(III) oxide. It is soluble in acids.[1][4][5]
Uses[edit]
Owing to its solubility, antimony sulfate has uses in the doping of semiconductors.[6] It is also used for coating anodes in electrolysis and in the production of explosives and fireworks.[4]
Safety[edit]
Antimony(III) sulfate causes irritation to the skin and mucous membranes.[7]
Natural occurrence[edit]
Natural analogue of the exact compound is yet unknown. However, basic hydrated Sb sulfates are known as the minerals klebelsbergite[8][9] and coquandite.[10][9]
References[edit]
- ^ a b c d e f R. Mercier; J. Douglade; J. Bernard (1976). "Structure cristalline de Sb2O3.3SO3". Acta Crystallographica Section B (in French). 32 (10): 2787–2791. doi:10.1107/S0567740876008881.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d Herbst, Karl Albert et al. (1985) Antimony and antimony compounds in Ullmann's Encyclopedia of Industrial Chemistry 5th ed., vol. A3, p. 70. ISBN 3-527-20103-3.
- ^ Nicholas C. Norman (31 December 1997). Chemistry of arsenic, antimony, and bismuth. Springer. pp. 193–. ISBN 978-0-7514-0389-3.
- ^ Method of forming phase change layer, method of manufacturing a storage node using the same, and method of manufacturing phase change memory device using the same – Samsung Electronics Co., Ltd. Freepatentsonline.com (2007-01-02). Retrieved on 2011-12-23.
- ^ Antimony(III) Sulfate Material Safety Data Sheet Archived 2012-04-26 at the Wayback Machine. Prochemonline.
- ^ "Klebelsbergite".
- ^ a b "List of Minerals". 21 March 2011.
- ^ "Coquandite".
