Caesium sulfate
| |

| |
| Names | |
|---|---|
| Other names
Cesium sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.589 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
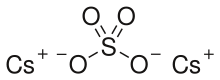
| Cs2SO4 | |
| Molar mass | 361.87 g/mol |
| Density | 4.243 g/cm3, solid |
| Melting point | 1,010 °C (1,850 °F; 1,280 K) |
| 167 g/100 ml (0 °C) 179 g/100 ml (20 °C) | |
| Solubility | insoluble in ethanol, acetone |
| -116.0·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H361, H412 | |
| P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P405, P501 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2830 mg/kg (oral, rat)[2] |
| Related compounds | |
Other anions
|
Caesium hydrogen sulfate |
Other cations
|
Lithium sulfate Sodium sulfate Potassium sulfate Rubidium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Caesium sulfate or cesium sulfate is the inorganic compound and salt with the formula Cs2SO4. It is a white water-soluble solid that is used to prepare dense aqueous solutions for use in isopycnic (or "density-gradient") centrifugation. It is isostructural with potassium salt.[3]
-
Coordination sphere of one of two types of Cs+ site in Cs2SO4.
-
Environment of sulfate anion in β-Cs2SO4.
References[edit]
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-92. ISBN 0-8493-0462-8..
- ^ https://chem.nlm.nih.gov/chemidplus/rn/10294-54-9 [dead link]
- ^ Nord, A.G. "The crystal structure of cesium sulfate, beta-Cs2SO4" Acta Chemica Scandinavica, Series A: 1976, 30, p198.


