Benazepril
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /bəˈnæzəprɪl/ |
| Trade names | Lotensin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692011 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 96.7% |
| Metabolism | Liver glucuronidation |
| Elimination half-life | 10-11 hours |
| Excretion | Kidney and bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
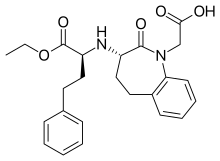
| Formula | C24H28N2O5 |
| Molar mass | 424.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[2] It is a reasonable initial treatment for high blood pressure.[2] It is taken by mouth.[2] Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.[2]
Common side effects include feeling tired, dizziness, cough, and light-headedness with standing.[2] Serious side effects may include kidney problems, low blood pressure, high blood potassium, and angioedema.[2] Use in pregnancy may harm the baby, while use when breastfeeding may be safe.[3] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[2]
Benazepril was patented in 1981 and came into medical use in 1990. It is available as a generic medication.[2] In 2021, it was the 147th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[4][5]
Medical uses[edit]
Lotensin is indicated for the treatment of hypertension, to lower blood pressure.[1][2]
Side effects[edit]
The most common side effects patients experience are a headache or a chronic cough. The chronic cough develops in about 20% of people treated.[6]
Contraindications[edit]
Benazepril can harm the fetus.[7]
Dosage forms[edit]
It is also available in combination with hydrochlorothiazide, under the brand name Lotensin HCT, and with amlodipine (Lotrel).
Veterinary uses[edit]
Under the brand names Fortekor (Novartis) and VetACE (Jurox Animal Health),[citation needed] benazepril is used to treat congestive heart failure in dogs[8][9] and chronic kidney failure in cats and dogs.[10]
References[edit]
- ^ a b "Lotensin- benazepril hydrochloride tablet". DailyMed. 21 January 2019. Retrieved 11 February 2024.
- ^ a b c d e f g h i "Benazepril Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ "Benazepril Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Benazepril - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ Dykewicz MS (April 2004). "Cough and Angioedema From Angiotensin-Converting Enzyme Inhibitors: New Insights Into Mechanisms and Management". Medscape. Retrieved 2 April 2014.
- ^ "Lotensin package insert" (PDF). U.S. Food and Drug Administration (FDA). 2011. Retrieved 9 December 2020.
- ^ King JN, Mauron C, Kaiser G (December 1995). "Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs". American Journal of Veterinary Research. 56 (12): 1620–1628. PMID 8599524.
- ^ O'Grady MR, O'Sullivan ML, Minors SL, Horne R (2009). "Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers". Journal of Veterinary Internal Medicine. 23 (5): 977–983. doi:10.1111/j.1939-1676.2009.0346.x. PMID 19572914.
- ^ "Fortekor Flavor Tabs (5 mg) (Canada) for Animal Use". Drugs.com. Retrieved 9 December 2020.
