Losartan
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /loʊˈsɑːrtən/ |
| Trade names | Cozaar, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695008 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Angiotensin II receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 25–35% |
| Protein binding | 99.7% (primarily albumin) |
| Metabolism | Liver (CYP2C9, CYP3A4) |
| Elimination half-life | 1.5–2 hours |
| Excretion | Kidney 13–25%, bile duct 50–60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.555 |
| Chemical and physical data | |
| Formula | C22H23ClN6O |
| Molar mass | 422.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension).[4] It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besides hypertension, it is also used in diabetic kidney disease, heart failure, and left ventricular enlargement.[4] It comes as a tablet that is taken by mouth.[4] It may be used alone or in addition to other blood pressure medication.[4] Up to six weeks may be required for the full effects to occur.[4]
Common adverse effects include muscle cramps, stuffy nose, dizziness, cough, high blood potassium, and anemia.[4] Severe adverse effects may include angioedema, low blood pressure, and kidney problems.[4] Use during pregnancy may result in harm to the baby.[4][1] Use is not recommended during breastfeeding.[1] It works by blocking angiotensin II.[4]
Losartan was patented in 1986, and approved for medical use in the United States in 1995.[4][5] It is on the World Health Organization's List of Essential Medicines.[6] It is available as a generic medication.[7] In 2022, it was the eighth most commonly prescribed medication in the United States, with more than 53 million prescriptions.[8][9] A version combined with hydrochlorothiazide is available[4] which, in 2022, was the 75th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[8][10]
Chemistry
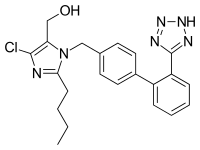
[edit]Losartan potassium is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt. Its empirical formula is C22H23CIKN6O , and its molecular weight is 422.9.[11]
Losartan is generally marketed as the (basic) potassium salt of the aromatized negatively charged tetrazole, called "losartan potassium".[12] The molecule has an extended biphenyl group with a tetrazole which is being used in place of the carboxylic acid as a bioisostere.[13]
Medical uses
[edit]Losartan is used for hypertension, including in people with left ventricular hypertrophy (enlarged heart muscle), and kidney dysfunction among type II diabetics.[3] It may also delay progression of diabetic nephropathy. It is a suitable pharmacological agent for the reduction of renal disease progression in patients with type 2 diabetes, hypertension, and microalbuminuria (>30 mg/24 hours) or proteinuria (>900 mg/24 hours).[14]
Although evidence shows calcium channel blockers and thiazide-type diuretics are preferred first-line treatments for most people (due to both efficacy and cost), an angiotensin II receptor antagonist such as losartan is recommended as first-line treatment in people under the age of 55 who cannot tolerate an ACE inhibitor.[15] One study demonstrated losartan was superior to atenolol in the primary prevention of adverse cardiovascular events (myocardial infarction or stroke), with a reduction in cardiovascular morbidity and mortality for a comparable reduction in blood pressure. The maximal effects on blood pressure usually occur within 3–6 weeks of starting losartan.[16]
Adverse effects
[edit]The most common adverse effects for losartan in adults are upper respiratory infections, dizziness, and back pain.[3] People with type 2 diabetes and kidney disease may experience diarrhea, fatigue, low blood pressure, low blood glucose, elevated potassium, chest pain, or allergic reaction.[3] Losartan should not be taken by people who are diabetic and taking aliskiren.[3] Anemia may occur, due to inhibition of the renin–angiotensin system.[17] As with other angiotensin receptor blockers, losartan may injure the liver, although this effect appears to be rare.[18] Electrolyte imbalances may occur in people with kidney problems who take losartan.[3] Adverse outcomes do not differ by sex, age or race.[3]
Pregnancy
[edit]In October 2014, the U.S. Food and Drug Administration (FDA) issued a black box warning that losartan can cause fetal toxicity, and should be discontinued as soon as pregnancy is detected.[19][3] Using losartan while pregnant could result in fetal injury or death.[19][3]
Overdose
[edit]Overdosing would most likely result in decreased blood pressure, which could manifest as an increased heart rate, dizziness, feeling light headed, or loss of consciousness. Mice studies showed that lethality occurred at about 44 to 170 times the maximum recommended dose after the mice weights were taken into account.[3]
Interactions
[edit]Losartan may have adverse interactions with phenobarbital, rifampin, or fluconazole, possibly inhibiting its blood pressure-lowering effects.[3]
Contamination
[edit]Between November 2018 and September 2019, the FDA announced multiple recalls of tablets containing losartan by Sandoz, Torrent Pharmaceuticals, Hetero Labs, Camber Pharmaceuticals, Legacy Pharmaceutical Packaging, Teva Pharmaceuticals, Vivimed Life Sciences, and Macleods Pharmaceutical Limited due to detection of one of the possible carcinogens N-nitrosodiethylamine, N-methylnitrosobutyric acid, or N-nitroso-N-methyl-4-aminobutyric acid in the active pharmaceutical ingredient (API).[20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37]
Mechanism of action
[edit]
Losartan is a selective, competitive angiotensin II receptor type 1 (AT1) antagonist, reducing the end organ responses to angiotensin II. Losartan administration results in a decrease in total peripheral resistance (afterload) and cardiac venous return (preload). All of the physiological effects of angiotensin II, including release of aldosterone, are antagonized in the presence of losartan. Reduction in blood pressure occurs independently of the status of the renin–angiotensin system. As a result of losartan dosing, plasma renin activity increases due to removal of the angiotensin II feedback. Renin is released from the kidneys when there is reduced renal arterial pressure, sympathetic activation, or increased sodium delivery to the distal renal tubule.[38] Renin then acts by converting angiotensinogen to angiotensin I; angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II; angiotensin II causes vasoconstriction and aldosterone release.[38] Aldosterone serves to retain sodium from the distal renal tubule. Sodium retention ultimately results in increased blood pressure.[39] Therefore, the use of angiotensin II receptor antagonists like losartan result in blocking the downstream effect of renin, angiotensin II, and ultimately decreasing blood pressure.
Angiotensin II receptor antagonists include losartan, valsartan, azilsartan, candesartan, eprosartan, irbesartan, olmesartan, and telmisartan. They all have the same mechanism of action and potentially inhibit the actions of angiotensin better than ACE inhibitors, such as lisinopril, because there are other enzymes than ACE that have the capability of producing angiotensin II.[38]
Losartan is a uricosuric. As a specific inhibitor of the urate transporter 1 (SLC22A12, URAT1), losartan blocks the uptake of uric acid into cells, thus leaving more available in the bloodstream to be filtered and excreted by the kidneys.[40] Because losartan can cause hyperkalemia, individuals should not use potassium supplements or salt substitutes containing potassium without appropriate monitoring by a physician.[41]
Pharmacokinetics
[edit]Losartan is well absorbed following oral administration and undergoes significant first-pass metabolism to produce the 5-carboxylic acid metabolite, designated as EXP3174. About 14% of an oral dosage is converted to this metabolite, which is long-acting (6 to 8 hr) and a noncompetitive antagonist at the AT1 receptor, contributing to the pharmacological effects of losartan. EXP3174 is 10-40 times more potent in blocking AT1 receptors than losartan. In addition, the binding to the target enzyme is pH-sensitive, and the negatively-charged tetrazole ring, which is similar in size to the negative carboxylic acid derivative, may contribute to the activity of the drug.[42]
Losartan's bioavailability is about 33%.[43]
Metabolism is primarily by cytochrome P450 isoenzymes CYP2C9 and CYP3A4.[44] Peak plasma concentrations of losartan and EXP3174 occur about one hour and three to four hours, respectively, after an oral dose.[45] Both losartan and EXP3174 are more than 98% bound to plasma proteins.[46] Losartan is excreted in the urine, and in the feces via bile, as unchanged drug and metabolites.[47] About 4% of an oral dose is excreted unchanged in urine, and about 6% is excreted in urine as the active metabolite.[48] The terminal elimination half lives of losartan and EXP3174 are about 1.5 to 2.5 hours and 3 to 9 hours, respectively.[49]
Losartan and other angiotensin-receptor antagonists exhibit fetal toxicity and should be avoided during pregnancy, particularly in the second and third trimesters.[50]
History
[edit]References
[edit]- ^ a b c "Losartan (Cozaar) Use During Pregnancy". Drugs.com. Archived from the original on 10 December 2017. Retrieved 10 December 2017.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e f g h i j k "Cozaar- losartan potassium tablet, film coated". DailyMed. 14 November 2019. Archived from the original on 28 April 2021. Retrieved 20 March 2020.
- ^ a b c d e f g h i j k "Losartan Potassium". The American Society of Health-System Pharmacists. Archived from the original on 10 December 2017. Retrieved 8 December 2017.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 470. ISBN 9783527607495. Archived from the original on 28 August 2021. Retrieved 26 August 2020.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 127. ISBN 9780857111562.
- ^ a b "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Losartan Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Hydrochlorothiazide; Losartan Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Pharmaceutical formulation of losartan". Archived from the original on 5 January 2022. Retrieved 5 January 2022.
- ^ "See negatively charged tetrazole structure". Archived from the original on 22 January 2021. Retrieved 21 October 2017.
- ^ "DailyMed - Losartan Potassium 25 mg- losartan potassium tablet, film coated ; Losartan Potassium 50 mg- losartan potassium tablet, film coated ; Losartan Potassium 100 mg- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ Boersma C, Atthobari J, Gansevoort RT, de Jong-Van den Berg LT, de Jong PE, de Zeeuw D, et al. (2006). "Pharmacoeconomics of angiotensin II antagonists in type 2 diabetic patients with nephropathy: implications for decision making". PharmacoEconomics. 24 (6): 523–35. doi:10.2165/00019053-200624060-00001. PMID 16761901. S2CID 22960961.
- ^ "Hypertension in adults: diagnosis and management". National Institute for Health and Care Excellence (NICE). 24 August 2011. Archived from the original on 9 April 2017. Retrieved 8 April 2017.
- ^ Abrams A (2007). 'Clinical Drug Therapy Rationales for Nursing Practice. Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 846. ISBN 978-0-7817-6263-2.
- ^ Cheungpasitporn W, Thongprayoon C, Chiasakul T, Korpaisarn S, Erickson SB (November 2015). "Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis". QJM. 108 (11): 879–884. doi:10.1093/qjmed/hcv049. PMID 25697787.
- ^ Patti R, Sinha A, Sharma S, Yoon TS, Kupfer Y (May 2019). "Losartan-induced Severe Hepatic Injury: A Case Report and Literature Review". Cureus. 11 (5): e4769. doi:10.7759/cureus.4769. PMC 6663042. PMID 31363450.
- ^ a b "Cozaar (losartan potassium) 25 mg, 50 mg, and 100 mg Tablets". U.S. Food and Drug Administration (FDA). 16 October 2014. Archived from the original on 12 January 2017. Retrieved 21 July 2015.
- ^ "FDA provides update on its ongoing investigation into ARB drug products; reports on finding of a new nitrosamine impurity in certain lots of losartan and product recall" (Press release). U.S. Food and Drug Administration (FDA). 3 October 2019. Archived from the original on 3 October 2019. Retrieved 3 October 2019.
- ^ "Sandoz Inc. Issues Voluntary Nationwide Recall of One Lot of Losartan Potassium and Hydrochlorothiazide Due to the Detection of Trace Amounts of NDEA (N-Nitrosodiethylamine) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 8 November 2018. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP". U.S. Food and Drug Administration (FDA). 20 December 2018. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP". U.S. Food and Drug Administration (FDA). 3 January 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Updated: Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium and Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 22 January 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Updated: Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium /Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 1 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Updated: Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium/Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 18 April 2019. Archived from the original on 6 October 2019. Retrieved 5 October 2019.
- ^ "Updated: Torrent Pharmaceuticals Limited Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP and Losartan Potassium / Hydrochlorothiazide Tablets, USP". U.S. Food and Drug Administration (FDA). 23 September 2019. Archived from the original on 6 October 2019. Retrieved 23 September 2019.
- ^ "Legacy Pharmaceutical Packaging, LLC Expands Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 50mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 15 July 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- ^ "Macleods Pharmaceutical Limited Issues Voluntary Nationwide Consumer Level Recall of Losartan Potassium 50mg and Losartan Potassium/Hydrochlorothiazide combination Tablets 50mg/12.5mg, 100mg/12.5mg and 100mg/25mg due to detection of NMBA (N-Nitroso-N-Methyl-4-aminobutyric acid) Impurity". U.S. Food and Drug Administration (FDA). 26 June 2019. Archived from the original on 1 October 2019. Retrieved 5 October 2019.
- ^ "Teva Pharmaceuticals USA, Inc. Expands Voluntary Nationwide Recall of Losartan Potassium to 50 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply, Inc". U.S. Food and Drug Administration (FDA). 11 June 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- ^ "Vivimed Life Sciences Pvt Ltd Issues Voluntary Nationwide Recall of Losartan Potassium 25 mg, 50 mg and 100 mg Tablets, USP Due to the Detection of Trace Amounts of N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) Impurity". U.S. Food and Drug Administration (FDA). 3 May 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- ^ "Teva Pharmaceuticals USA, Inc. Issues Voluntary Nationwide Recall of Losartan Potassium 25 mg and 100 mg Tablets USP, Sold Exclusively to Golden State Medical Supply". U.S. Food and Drug Administration (FDA). 26 April 2019. Archived from the original on 13 September 2019. Retrieved 5 October 2019.
- ^ "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25mg, 50mg, And 100mg Due to The Detection of Trace Amounts Of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) Impurity Found in The Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 19 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25mg, 50mg, and 100mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) Impurity Found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 28 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 50mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 15 March 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Camber Pharmaceuticals, Inc. Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25 mg, 50 mg and 100 mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) Impurity found in the Active Pharmaceutical Ingredient (API)". U.S. Food and Drug Administration (FDA). 28 February 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ "Macleods Pharmaceuticals Limited Issues Voluntary Nationwide Consumer Level Recall of One Lot (BLM 715A) of Losartan Potassium/Hydrochlorothiazide Combination Tablets 100mg/25mg Due to detection of NDEA (N-Nitrosodiethylamine) Impurity". U.S. Food and Drug Administration (FDA). 22 February 2019. Archived from the original on 7 September 2019. Retrieved 5 October 2019.
- ^ a b c Katzung, Bertram G., ed. (30 November 2017). Basic & clinical pharmacology. McGraw-Hill Education. ISBN 9781259641152. OCLC 1048625746.
- ^ Graudal NA, Hubeck-Graudal T, Jürgens G (January 2012). "Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review)". American Journal of Hypertension. 25 (1): 1–15. doi:10.1038/ajh.2011.210. PMID 22068710.
- ^ Hamada T, Ichida K, Hosoyamada M, Mizuta E, Yanagihara K, et al. (1 October 2008). "Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients". American Journal of Hypertension. 21 (10): 1157–1162. doi:10.1038/ajh.2008.245. PMID 18670416.
- ^ RxList. The Internet Drug Index. Clinical pharmacology of Cozaar Archived 6 January 2014 at the Wayback Machine. Retrieved 6 January 2014.
- ^ Noda K, Saad Y, Kinoshita A, Boyle TP, Graham RM, Husain A, et al. (February 1995). "Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms". The Journal of Biological Chemistry. 270 (5): 2284–2289. doi:10.1074/jbc.270.5.2284. PMID 7530721. Archived from the original on 19 September 2022. Retrieved 21 October 2017.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ "DailyMed - LOSARTAN POTASSIUM 25 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 50 MG- losartan potassium tablet, film coated LOSARTAN POTASSIUM 100 MG- losartan potassium tablet, film coated". Archived from the original on 27 April 2022. Retrieved 27 April 2022.
- ^ Sica DA, Gehr TW, Ghosh S (2005). "Clinical pharmacokinetics of losartan". Clin Pharmacokinet. 44 (8): 797–814. doi:10.2165/00003088-200544080-00003. PMID 16029066. S2CID 41326620.
Further reading
[edit]- Al-Majed AR, Assiri E, Khalil NY, Abdel-Aziz HA (2015). "Losartan: Comprehensive Profile". Profiles Drug Subst Excip Relat Methodol. 40: 159–94. doi:10.1016/bs.podrm.2015.02.003. PMID 26051686.
- Sica DA, Gehr TW, Ghosh S (2005). "Clinical pharmacokinetics of losartan". Clin Pharmacokinet. 44 (8): 797–814. doi:10.2165/00003088-200544080-00003. PMID 16029066. S2CID 41326620.
External links
[edit] Media related to Losartan at Wikimedia Commons
Media related to Losartan at Wikimedia Commons- "Nitrosamine impurities in medications: Guidance". Health Canada. 4 April 2022.
