Chronic myelogenous leukemia
| Chronic myelogenous leukemia | |
|---|---|
| Specialty | Hematology |
Chronic myelogenous (or myeloid) leukemia (CML), also known as chronic granulocytic leukemia (CGL), is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which proliferation of mature granulocytes (neutrophils, eosinophils, and basophils) and their precursors is the main finding. It is a type of myeloproliferative disease associated with a characteristic chromosomal translocation called the Philadelphia chromosome. CML is now largely treated with tyrosine kinase inhibitors (TKIs), such as imatinib (Gleevec/Glivec), dasatinib, or nilotinib, which have led to dramatically improved survival rates since their introduction in the last decade.
Signs and symptoms
Patients are often asymptomatic at diagnosis, presenting incidentally with an elevated white blood cell count on a routine laboratory test. In this setting, CML must be distinguished from a leukemoid reaction, which can have a similar appearance on a blood smear. Symptoms of CML may include: enlarged spleen causing pain on the left side, malaise, joint and/or hip pain, low-grade fever, increased susceptibility to infections, anemia, and thrombocytopenia with easy bruising (although an increased platelet count (thrombocytosis) may also occur in CML).[1][2]
Diagnosis
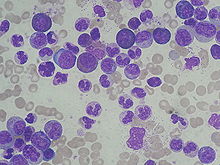

CML is often suspected on the basis on the complete blood count, which shows increased granulocytes of all types, typically including mature myeloid cells. Basophils and eosinophils are almost universally increased; this feature may help differentiate CML from a leukemoid reaction. A bone marrow biopsy is often performed as part of the evaluation for CML, and CML is diagnosed by detecting the Philadelphia chromosome. This characteristic chromosomal abnormality can be detected by routine cytogenetics, by fluorescent in situ hybridization, or by PCR for the bcr-abl fusion gene.[2]
Controversy exists over so-called Ph-negative CML, or cases of suspected CML in which the Philadelphia chromosome cannot be detected. Many such patients in fact have complex chromosomal abnormalities that mask the (9;22) translocation, or have evidence of the translocation by FISH or RT-PCR in spite of normal routine karyotyping.[3] The small subset of patients without detectable molecular evidence of bcr-abl fusion may be better classified as having an undifferentiated myelodysplastic/myeloproliferative disorder, as their clinical course tends to be different from patients with CML.[4]
Pathophysiology
CML was the first malignancy to be linked to a clear genetic abnormality, the chromosomal translocation known as the Philadelphia chromosome. This chromosomal abnormality is so named because it was first discovered and described in 1960 by two scientists from Philadelphia, Pennsylvania, USA: Peter Nowell of the University of Pennsylvania and David Hungerford of Fox Chase Cancer Center.[5]
In this translocation, parts of two chromosomes (the 9th and 22nd by conventional karyotypic numbering) switch places. As a result, part of the BCR ("breakpoint cluster region") gene from chromosome 22 is fused with the ABL gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185 weight (p210 is short for 210 kDa protein, a shorthand used for characterizing proteins based solely on size). Because abl carries a domain that can add phosphate groups to tyrosine residues (a tyrosine kinase), the bcr-abl fusion gene product is also a tyrosine kinase.[1][6]
The fused BCR-ABL protein interacts with the interleukin 3beta(c) receptor subunit. The BCR-ABL transcript is continuously active and does not require activation by other cellular messaging proteins. In turn, BCR-ABL activates a cascade of proteins that control the cell cycle, speeding up cell division. Moreover, the BCR-ABL protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the BCR-ABL protein is the pathophysiologic cause of chronic myelogenous leukemia. With improved understanding of the nature of the BCR-ABL protein and its action as a tyrosine kinase, targeted therapies (the first of which was imatinib mesylate) that specifically inhibit the activity of the BCR-ABL protein have been developed. These tyrosine kinase inhibitors can induce complete remissions in CML, confirming the central importance of bcr-abl as the cause of CML.[6]
Classification
CML is often divided into three phases based on clinical characteristics and laboratory findings. In the absence of intervention, CML typically begins in the chronic phase, and over the course of several years progresses to an accelerated phase and ultimately to a blast crisis. Blast crisis is the terminal phase of CML and clinically behaves like an acute leukemia. Drug treatment will usually stop this progression if started early. One of the drivers of the progression from chronic phase through acceleration and blast crisis is the acquisition of new chromosomal abnormalities (in addition to the Philadelphia chromosome).[1] Some patients may already be in the accelerated phase or blast crisis by the time they are diagnosed.[2]
Chronic phase
Approximately 85% of patients with CML are in the chronic phase at the time of diagnosis. During this phase, patients are usually asymptomatic or have only mild symptoms of fatigue, left side pain, joint and/or hip pain, or abdominal fullness. The duration of chronic phase is variable and depends on how early the disease was diagnosed as well as the therapies used. In the absence of treatment, the disease progresses to an accelerated phase.[2]
Accelerated phase
Criteria for diagnosing transition into the accelerated phase are somewhat variable; the most widely used criteria are those put forward by investigators at M.D. Anderson Cancer Center,[7] by Sokal et al.,[8] and the World Health Organization.[4][9] The WHO criteria are perhaps most widely used, and define the accelerated phase by any of the following:
- 10–19% myeloblasts in the blood or bone marrow
- >20% basophils in the blood or bone marrow
- Platelet count <100,000, unrelated to therapy
- Platelet count >1,000,000, unresponsive to therapy
- Cytogenetic evolution with new abnormalities in addition to the Philadelphia chromosome
- Increasing splenomegaly or white blood cell count, unresponsive to therapy
The patient is considered to be in the accelerated phase if any of the above are present. The accelerated phase is significant because it signals that the disease is progressing and transformation to blast crisis is imminent. Drug treatment often becomes less effective in the advanced stages.[4]
Blast crisis
Blast crisis is the final phase in the evolution of CML, and behaves like an acute leukemia, with rapid progression and short survival.[2] Blast crisis is diagnosed if any of the following are present in a patient with CML:[10]
- >20% myeloblasts or lymphoblasts in the blood or bone marrow
- Large clusters of blasts in the bone marrow on biopsy
- Development of a chloroma (solid focus of leukemia outside the bone marrow)
Treatment
Chronic phase
Chronic phase CML is treated with inhibitors of tyrosine kinase, the first of which was imatinib mesylate (marketed as Gleevec or Glivec; previously known as STI-571 or CGP57148B) approved by the United States FDA in 2001. Imatinib has proven to inhibit the progression of CML, allowing the majority of patients (65–75%) to have a regrowth of their normal bone marrow (cytogenetic response). Although few leukemic cells persist in most patients as evaluated by RT-PCR, and treatment has to be continued indefinitely, CML is the first cancer in which a medical treatment (imatinib) can give to the treated patients a normal life expectancy.[11] To overcome imatinib resistance and to increase responsiveness of TK inhibitors, two novel agents were later developed. The first, dasatinib, is a TK inhibitor that blocks several oncogenic proteins and was initially approved by the US FDA in 2007 to treat CML patients who were either resistant to or intolerant of imatinib. Another TK inhibitor, nilotinib, was also approved by the US FDA for the same indication. Nilotinib and dasatanib were approved for first-line therapy in 2010, so there are now three drugs available for first-line treatment of CML. In the past, antimetabolites (e.g., cytarabine, hydroxyurea), alkylating agents, interferon alfa 2b, and steroids were used, but these drugs have been replaced by TK inhibitor drugs. The TK inhibitor drugs specifically target BCR/abl, the constitutively activated tyrosine kinase fusion protein caused by the Philadelphia chromosome translocation. The IRIS study is an international study that compared interferon/cytarabine combination with imatinib. Long-term follow up demonstrating the superiority of imatinib regimens is clear-cut. Bone marrow transplantation was also used as initial treatment for CML before the advent of imatinib, but is now rarely used, primarily for those who fail drug treatment. While transplantation can often be curative, there remains a high rate of transplant-related mortality.[citation needed]
Dasatanib and nilotinib failed to overcome the imatinib resistance caused by the T315I mutation. All current treatments for this mutation are experimental. In October 2010, ponatinib, a small molecule, oral drug developed by Ariad Pharmaceuticals, was entered into evaluation in a pivotal Phase 2 trial. Ponatinib, a pan-BCR-ABL inhibitor, has shown ongoing strong efficacy in its continuing Phase 1 trial in treatment of the T315I mutation CML, as well as all other known mutations. This drug has also been granted orphan status. Recently Chemgenex released results of their open-label Phase 2/3 study (CGX-635-CML-202) which investigated the use of their non-targeted agent omacetaxine, administered subcutaneously in CML patients who had failed imatinib and who have the highly drug-resistant T315I kinase domain mutation. Stem cell transplantation remains an option for those patients who rarely develop the T315I mutation.[12][13]
In 2005, encouraging but mixed results of vaccination were reported with the BCR/abl p210 fusion protein in patients with stable disease, with GM-CSF as an adjuvant.[14]
Prognosis
A follow-up on patients using imatinib published in the New England Journal of Medicine in 2006 showed an overall survival rate of 89% after five years.[15] In 2011, an independent study performed in 832 CML patients worldwide reported that the group of patients who achieve a stable cytogenetic response with imatinib shows an overall survival rate of 95.2% after 8 years, which is similar to the rate in the general population. Only 1% of patients died because of leukemia progression.[11]
Epidemiology
CML occurs in all age groups, but most commonly in the middle-aged and elderly. Its annual incidence is 1–2 per 100,000 people, and slightly more men than women are affected. CML represents about 15–20% of all cases of adult leukemia in Western populations.[1] The only well-described risk factor for CML is exposure to ionizing radiation; for example, increased rates of CML were seen in people exposed to the atomic bombings of Hiroshima and Nagasaki[16]
Leukemia is also rarely associated with pregnancy, affecting only about 1 in 10,000 pregnant women.[17] Chronic myelogenous leukemia can be treated with relative safety at any time during pregnancy with Interferon-alpha hormones.[17]
References
- ^ a b c d Faderl S, Talpaz M, Estrov Z, Kantarjian HM (1999). "Chronic myelogenous leukemia: biology and therapy". Annals of Internal Medicine. 131 (3): 207–219. PMID 10428738.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Tefferi A (2006). "Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era". Hematology Am Soc Hematol Educ Program. 2006: 240–5. doi:10.1182/asheducation-2006.1.240. PMID 17124067.
- ^ Savage DG; Szydlo RM; Goldman JM (1997). "Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period". Br J Haematol. 96 (1): 111–6. doi:10.1046/j.1365-2141.1997.d01-1982.x. PMID 9012696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, Barosi G, Verstovsek S, Birgegard G, Mesa R, Reilly JT, Gisslinger H, Vannucchi AM, Cervantes F, Finazzi G, Hoffman R, Gilliland DG, Bloomfield CD, Vardiman JW (2007). "Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert pane". Blood. 110 (4): 1092–7. doi:10.1182/blood-2007-04-083501. PMID 17488875.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nowell PC (2007). "Discovery of the Philadelphia chromosome: a personal perspective". Journal of Clinical Investigation. 117 (8): 2033–5. doi:10.1172/JCI31771. PMC 1934591. PMID 17671636.
- ^ a b Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. doi:10.1016/S0140-6736(07)61165-9. PMID 17662883.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kantarjian H, Dixon D, Keating M, Talpaz M, Walters R, McCredie K, Freireich E (1988). "Characteristics of accelerated disease in chronic myelogenous leukemia". Cancer. 61 (7): 1441–6. doi:10.1002/1097-0142(19880401)61:7<1441::AID-CNCR2820610727>3.0.CO;2-C. PMID 3162181.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sokal J, Baccarani M, Russo D, Tura S (1988). "Staging and prognosis in chronic myelogenous leukemia". Semin Hematol. 25 (1): 49–61. PMID 3279515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vardiman J, Harris N, Brunning R (2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood. 100 (7): 2292–302. doi:10.1182/blood-2002-04-1199. PMID 12239137. Retrieved 2007-09-22.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Karbasian Esfahani M, Morris EL, Dutcher JP, Wiernik PH (2006). "Blastic phase of chronic myelogenous leukemia". Current Treatment Options in Oncology. 7 (3): 189–199. doi:10.1007/s11864-006-0012-y. PMID 16615875.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21422402, please use {{cite journal}} with
|pmid=21422402instead. - ^ Jabbour E, Cortes JE, Giles FJ, O'Brien S, Kantarjian HM (2007). "Current and emerging treatment options in chronic myeloid leukemia". Cancer. 109 (11): 2171–81. doi:10.1002/cncr.22661. PMID 17431887.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kimura S, Ashihara E, Maekawa T (2006). "New tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia". Current Pharmaceutical Biotechnology. 7 (5): 371–9. doi:10.2174/138920106778521532. PMID 17076652.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Gozzetti A, Raspadori D, Amadori S, Lauria F (2005). "Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial". Lancet. 365 (9460): 657–62. doi:10.1016/S0140-6736(05)17945-8. PMID 15721470.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Druker BJ, Guilhot F, O'Brien SG; et al. (2006). "Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia". New England Journal of Medicine. 355 (20): 2408–17. doi:10.1056/NEJMoa062867. PMID 17151364.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Moloney WC (1987). "Radiogenic leukemia revisited". Blood. 70 (4): 905–8. PMID 3477299.
- ^ a b Shapira T, Pereg D, Lishner M (2008). "How I treat acute and chronic leukemia in pregnancy". Blood Rev. 22 (5): 247–59. doi:10.1016/j.blre.2008.03.006. PMID 18472198.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- 50th Anniversary Symposium of Discovery (US)
- The National CML Society (US)
- CMLeukemia
- Chronic Myeloid Leukemia at American Cancer Society
- CML information from The Leukemia & Lymphoma Society
- Chronic Myelocytic Leukemia (CML) at Merck Manual of Diagnosis and Therapy Home Edition
- SPIRIT 2 Website
