Diabetes
| Diabetes | |
|---|---|
| Specialty | Diabetology |
Diabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced.[2] This high blood sugar produces the classical symptoms of polyuria (frequent urination), polydipsia (increased thirst) and polyphagia (increased hunger).
The three main types of diabetes mellitus (DM) are:
- Type 1 DM results from the body's failure to produce insulin, and presently requires the person to inject insulin. (Also referred to as insulin-dependent diabetes mellitus (IDDM) or "juvenile" diabetes)
- Type 2 DM results from insulin resistance, a condition in which cells fail to use insulin properly, sometimes combined with an absolute insulin deficiency. (Formerly referred to as noninsulin-dependent diabetes mellitus (NIDDM) or "adult-onset" diabetes)
- Gestational diabetes is when pregnant women, who have never had diabetes before, have a high blood glucose level during pregnancy. It may precede development of type 2 DM.
Other forms of diabetes mellitus include congenital diabetes, which is due to genetic defects of insulin secretion, cystic fibrosis-related diabetes, steroid diabetes induced by high doses of glucocorticoids, and several forms of monogenic diabetes.
All forms of diabetes have been treatable since insulin became available in 1921, and type 2 diabetes may be controlled with medications. Both types 1 and 2 are chronic conditions that usually cannot be cured. Pancreas transplants have been tried with limited success in type 1 DM; gastric bypass surgery has been successful in many with morbid obesity and type 2 DM. Gestational diabetes usually resolves after delivery. Diabetes without proper treatments can cause many complications. Acute complications include hypoglycemia, diabetic ketoacidosis, or nonketotic hyperosmolar coma. Serious long-term complications include cardiovascular disease, chronic renal failure, and diabetic retinopathy (retinal damage). Adequate treatment of diabetes is thus important, as well as blood pressure control and lifestyle factors such as smoking cessation and maintaining a healthy body weight.
Globally, as of 2010[update], an estimated 285 million people have type 2 diabetes, making up about 90 percent of all diabetes cases.[3]
Classification
| Feature | Type 1 diabetes | Type 2 diabetes |
|---|---|---|
| Onset | Sudden | Gradual |
| Age at onset | Mostly in children | Mostly in adults |
| Body habitus | Thin or normal[4] | Often obese |
| Ketoacidosis | Common | Rare |
| Autoantibodies | Usually present | Absent |
| Endogenous insulin | Low or absent | Normal, decreased or increased |
| Concordance in identical twins |
50% | 90% |
| Prevalence | ~10% | ~90% |
Diabetes mellitus is classified into four broad categories: type 1, type 2, gestational diabetes and "other specific types".[2] The "other specific types" are a collection of a few dozen individual causes.[2] The term "diabetes", without qualification, usually refers to diabetes mellitus. The rare disease diabetes insipidus has similar symptoms as diabetes mellitus, but without disturbances in the sugar metabolism (insipidus means "without taste" in Latin).
The term "type 1 diabetes" has replaced several former terms, including childhood-onset diabetes, juvenile diabetes, and insulin-dependent diabetes mellitus (IDDM). Likewise, the term "type 2 diabetes" has replaced several former terms, including adult-onset diabetes, obesity-related diabetes, and noninsulin-dependent diabetes mellitus (NIDDM). Beyond these two types, there is no agreed-upon standard nomenclature.
Type 1 diabetes
Type 1 diabetes mellitus is characterized by loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas, leading to insulin deficiency. This type can be further classified as immune-mediated or idiopathic. The majority of type 1 diabetes is of the immune-mediated nature, in which beta cell loss is a T-cell-mediated autoimmune attack.[5] There is no known preventive measure against type 1 diabetes, which causes approximately 10% of diabetes mellitus cases in North America and Europe. Most affected people are otherwise healthy and of a healthy weight when onset occurs. Sensitivity and responsiveness to insulin are usually normal, especially in the early stages. Type 1 diabetes can affect children or adults, but was traditionally termed "juvenile diabetes" because a majority of these diabetes cases were in children.
"Brittle" diabetes, also known as unstable diabetes or labile diabetes, is a term that was traditionally used to describe to dramatic and recurrent swings in glucose levels, often occurring for no apparent reason in insulin-dependent diabetes. This term, however, has no biologic basis and should not be used.[6] There are many different reasons for type 1 diabetes to be accompanied by irregular and unpredictable hyperglycemias, frequently with ketosis, and sometimes serious hypoglycemias, including an impaired counterregulatory response to hypoglycemia, occult infection, gastroparesis (which leads to erratic absorption of dietary carbohydrates), and endocrinopathies (e.g., Addison's disease).[6] These phenomena are believed to occur no more frequently than in 1% to 2% of persons with type 1 diabetes.[7]
Type 2 diabetes
Type 2 diabetes mellitus is characterized by insulin resistance, which may be combined with relatively reduced insulin secretion.[2] The defective responsiveness of body tissues to insulin is believed to involve the insulin receptor. However, the specific defects are not known. Diabetes mellitus cases due to a known defect are classified separately. Type 2 diabetes is the most common type.
In the early stage of type 2, the predominant abnormality is reduced insulin sensitivity. At this stage, hyperglycemia can be reversed by a variety of measures and medications that improve insulin sensitivity or reduce glucose production by the liver.
Gestational diabetes
Gestational diabetes mellitus (GDM) resembles type 2 diabetes in several respects, involving a combination of relatively inadequate insulin secretion and responsiveness. It occurs in about 2%–5% of all pregnancies and may improve or disappear after delivery. Gestational diabetes is fully treatable, but requires careful medical supervision throughout the pregnancy. About 20%–50% of affected women develop type 2 diabetes later in life.
Though it may be transient, untreated gestational diabetes can damage the health of the fetus or mother. Risks to the baby include macrosomia (high birth weight), congenital cardiac and central nervous system anomalies, and skeletal muscle malformations. Increased fetal insulin may inhibit fetal surfactant production and cause respiratory distress syndrome. Hyperbilirubinemia may result from red blood cell destruction. In severe cases, perinatal death may occur, most commonly as a result of poor placental perfusion due to vascular impairment. Labor induction may be indicated with decreased placental function. A Caesarean section may be performed if there is marked fetal distress or an increased risk of injury associated with macrosomia, such as shoulder dystocia.
A 2008 study completed in the U.S. found the number of American women entering pregnancy with pre-existing diabetes is increasing. In fact, the rate of diabetes in expectant mothers has more than doubled in the past six years.[8] This is particularly problematic as diabetes raises the risk of complications during pregnancy, as well as increasing the potential for the children of diabetic mothers to become diabetic in the future.
Other types
Prediabetes indicates a condition that occurs when a person's blood glucose levels are higher than normal but not high enough for a diagnosis of type 2 DM. Many people destined to develop type 2 DM spend many years in a state of prediabetes which has been termed "America's largest healthcare epidemic."[9]: 10–11
Latent autoimmune diabetes of adults (LADA) is a condition in which type 1 DM develops in adults. Adults with LADA are frequently initially misdiagnosed as having type 2 DM, based on age rather than etiology.
Some cases of diabetes are caused by the body's tissue receptors not responding to insulin (even when insulin levels are normal, which is what separates it from type 2 diabetes); this form is very uncommon. Genetic mutations (autosomal or mitochondrial) can lead to defects in beta cell function. Abnormal insulin action may also have been genetically determined in some cases. Any disease that causes extensive damage to the pancreas may lead to diabetes (for example, chronic pancreatitis and cystic fibrosis). Diseases associated with excessive secretion of insulin-antagonistic hormones can cause diabetes (which is typically resolved once the hormone excess is removed). Many drugs impair insulin secretion and some toxins damage pancreatic beta cells. The ICD-10 (1992) diagnostic entity, malnutrition-related diabetes mellitus (MRDM or MMDM, ICD-10 code E12), was deprecated by the World Health Organization when the current taxonomy was introduced in 1999.[10]
Signs and symptoms

The classical symptoms of untreated diabetes are loss of weight, polyuria (frequent urination), polydipsia (increased thirst) and polyphagia (increased hunger).[11] Symptoms may develop rapidly (weeks or months) in type 1 diabetes, while they usually develop much more slowly and may be subtle or absent in type 2 diabetes.
Prolonged high blood glucose can cause glucose absorption in the lens of the eye, which leads to changes in its shape, resulting in vision changes. Blurred vision is a common complaint leading to a diabetes diagnosis; type 1 should always be suspected in cases of rapid vision change, whereas with type 2 change is generally more gradual, but should still be suspected [citation needed]. A number of skin rashes that can occur in diabetes are collectively known as diabetic dermadromes.
Diabetic emergencies
People (usually with type 1 diabetes) may also present with diabetic ketoacidosis, a state of metabolic dysregulation characterized by the smell of acetone, a rapid, deep breathing known as Kussmaul breathing, nausea, vomiting and abdominal pain, and altered states of consciousness.
A rare but equally severe possibility is hyperosmolar nonketotic state, which is more common in type 2 diabetes and is mainly the result of dehydration.
Complications
All forms of diabetes increase the risk of long-term complications. These typically develop after many years (10–20), but may be the first symptom in those who have otherwise not received a diagnosis before that time. The major long-term complications relate to damage to blood vessels. Diabetes doubles the risk of cardiovascular disease.[12] The main "macrovascular" diseases (related to atherosclerosis of larger arteries) are ischemic heart disease (angina and myocardial infarction), stroke and peripheral vascular disease.
Diabetes also causes "microvascular" complications—damage to the small blood vessels.[13] Diabetic retinopathy, which affects blood vessel formation in the retina of the eye, can lead to visual symptoms, reduced vision, and potentially blindness. Diabetic nephropathy, the impact of diabetes on the kidneys, can lead to scarring changes in the kidney tissue, loss of small or progressively larger amounts of protein in the urine, and eventually chronic kidney disease requiring dialysis. Diabetic neuropathy is the impact of diabetes on the nervous system, most commonly causing numbness, tingling and pain in the feet and also increasing the risk of skin damage due to altered sensation. Together with vascular disease in the legs, neuropathy contributes to the risk of diabetes-related foot problems (such as diabetic foot ulcers) that can be difficult to treat and occasionally require amputation.
Causes
The cause of diabetes depends on the type.
Type 1 diabetes is partly inherited, and then triggered by certain infections, with some evidence pointing at Coxsackie B4 virus. A genetic element in individual susceptibility to some of these triggers has been traced to particular HLA genotypes (i.e., the genetic "self" identifiers relied upon by the immune system). However, even in those who have inherited the susceptibility, type 1 DM seems to require an environmental trigger.
Type 2 diabetes is due primarily to lifestyle factors and genetics.[14]
The following is a comprehensive list of other causes of diabetes:[15]
|
|
Pathophysiology
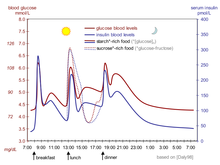

Insulin is the principal hormone that regulates uptake of glucose from the blood into most cells (primarily muscle and fat cells, but not central nervous system cells). Therefore, deficiency of insulin or the insensitivity of its receptors plays a central role in all forms of diabetes mellitus.
Humans are capable of digesting some carbohydrates, in particular those most common in food; starch, and some disaccharides such as sucrose, are converted within a few hours to simpler forms, most notably the monosaccharide glucose, the principal carbohydrate energy source used by the body. The rest are passed on for processing by gut flora largely in the colon. Insulin is released into the blood by beta cells (β-cells), found in the islets of Langerhans in the pancreas, in response to rising levels of blood glucose, typically after eating. Insulin is used by about two-thirds of the body's cells to absorb glucose from the blood for use as fuel, for conversion to other needed molecules, or for storage.
Insulin is also the principal control signal for conversion of glucose to glycogen for internal storage in liver and muscle cells. Lowered glucose levels result both in the reduced release of insulin from the β-cells and in the reverse conversion of glycogen to glucose when glucose levels fall. This is mainly controlled by the hormone glucagon, which acts in the opposite manner to insulin. Glucose thus forcibly produced from internal liver cell stores (as glycogen) re-enters the bloodstream; muscle cells lack the necessary export mechanism. Normally, liver cells do this when the level of insulin is low (which normally correlates with low levels of blood glucose).
Higher insulin levels increase some anabolic ("building up") processes, such as cell growth and duplication, protein synthesis, and fat storage. Insulin (or its lack) is the principal signal in converting many of the bidirectional processes of metabolism from a catabolic to an anabolic direction, and vice versa. In particular, a low insulin level is the trigger for entering or leaving ketosis (the fat-burning metabolic phase).
If the amount of insulin available is insufficient, if cells respond poorly to the effects of insulin (insulin insensitivity or resistance), or if the insulin itself is defective, then glucose will not have its usual effect, so it will not be absorbed properly by those body cells that require it, nor will it be stored appropriately in the liver and muscles. The net effect is persistent high levels of blood glucose, poor protein synthesis, and other metabolic derangements, such as acidosis.
When the glucose concentration in the blood is raised beyond its renal threshold (about 10 mmol/L, although this may be altered in certain conditions, such as pregnancy), reabsorption of glucose in the proximal renal tubuli is incomplete, and part of the glucose remains in the urine (glycosuria). This increases the osmotic pressure of the urine and inhibits reabsorption of water by the kidney, resulting in increased urine production (polyuria) and increased fluid loss. Lost blood volume will be replaced osmotically from water held in body cells and other body compartments, causing dehydration and increased thirst.
Diagnosis
| Condition | 2-hour glucose | Fasting glucose | HbA1c | |||
|---|---|---|---|---|---|---|
| Unit | mmol/L | mg/dL | mmol/L | mg/dL | mmol/mol | DCCT % |
| Normal | < 7.8 | < 140 | < 6.1 | < 110 | < 42 | < 6.0 |
| Impaired fasting glycaemia | < 7.8 | < 140 | 6.1–7.0 | 110–125 | 42–46 | 6.0–6.4 |
| Impaired glucose tolerance | ≥ 7.8 | ≥ 140 | < 7.0 | < 126 | 42–46 | 6.0–6.4 |
| Diabetes mellitus | ≥ 11.1 | ≥ 200 | ≥ 7.0 | ≥ 126 | ≥ 48 | ≥ 6.5 |
Diabetes mellitus is characterized by recurrent or persistent hyperglycemia, and is diagnosed by demonstrating any one of the following:[10]
- Fasting plasma glucose level ≥ 7.0 mmol/l (126 mg/dl)
- Plasma glucose ≥ 11.1 mmol/l (200 mg/dL) two hours after a 75 g oral glucose load as in a glucose tolerance test
- Symptoms of hyperglycemia and casual plasma glucose ≥ 11.1 mmol/l (200 mg/dl)
- Glycated hemoglobin (Hb A1C) ≥ 6.5%[19]
A positive result, in the absence of unequivocal hyperglycemia, should be confirmed by a repeat of any of the above methods on a different day. It is preferable to measure a fasting glucose level because of the ease of measurement and the considerable time commitment of formal glucose tolerance testing, which takes two hours to complete and offers no prognostic advantage over the fasting test.[20] According to the current definition, two fasting glucose measurements above 126 mg/dl (7.0 mmol/l) is considered diagnostic for diabetes mellitus.
People with fasting glucose levels from 110 to 125 mg/dl (6.1 to 6.9 mmol/l) are considered to have impaired fasting glucose.[21] Patients with plasma glucose at or above 140 mg/dL (7.8 mmol/L), but not over 200 mg/dL (11.1 mmol/L), two hours after a 75 g oral glucose load are considered to have impaired glucose tolerance. Of these two prediabetic states, the latter in particular is a major risk factor for progression to full-blown diabetes mellitus, as well as cardiovascular disease.[22]
Glycated hemoglobin is better than fasting glucose for determining risks of cardiovascular disease and death from any cause.[23]
Management
Diabetes mellitus is a chronic disease which cannot be cured except in very specific situations. Management concentrates on keeping blood sugar levels as close to normal ("euglycemia") as possible, without causing hypoglycemia. This can usually be accomplished with diet, exercise, and use of appropriate medications (insulin in the case of type 1 diabetes, oral medications, as well as possibly insulin, in type 2 diabetes).
Patient education, understanding, and participation is vital, since the complications of diabetes are far less common and less severe in people who have well-managed blood sugar levels.[24][25] The goal of treatment is an HbA1C level of 6.5%, but should not be lower than that, and may be set higher.[26] Attention is also paid to other health problems that may accelerate the deleterious effects of diabetes. These include smoking, elevated cholesterol levels, obesity, high blood pressure, and lack of regular exercise.[26]
Lifestyle
There are roles for patient education, dietetic support, sensible exercise, with the goal of keeping both short-term and long-term blood glucose levels within acceptable bounds. In addition, given the associated higher risks of cardiovascular disease, lifestyle modifications are recommended to control blood pressure.[27]
Medications
- Oral medications
Metformin is generally recommended as a first line treatment for type 2 diabetes, as there is good evidence that it decreases mortality.[28] Routine use of aspirin, however, has not been found to improve outcomes in uncomplicated diabetes.[29]
- Insulin
Type 1 diabetes is typically treated with a combinations of regular and NPH insulin, or synthetic insulin analogs. When insulin is used in type 2 diabetes, a long-acting formulation is usually added initially, while continuing oral medications.[28] Doses of insulin are then increased to effect.[28]
Support
In countries using a general practitioner system, such as the United Kingdom, care may take place mainly outside hospitals, with hospital-based specialist care used only in case of complications, difficult blood sugar control, or research projects. In other circumstances, general practitioners and specialists share care of a patient in a team approach. Optometrists, podiatrists/chiropodists, dietitians, physiotherapists, nursing specialists (e.g., diabetic specialist nurses), nurse practitioners, or certified diabetes educators, may jointly provide multidisciplinary expertise.[citation needed] Home telehealth support can be an effective management technique.[30]
Epidemiology


Globally, as of 2010[update], an estimated 285 million people had diabetes, with type 2 making up about 90% of the cases.[3] Its incidence is increasing rapidly, and by 2030, this number is estimated to almost double.[31] Diabetes mellitus occurs throughout the world, but is more common (especially type 2) in the more developed countries. The greatest increase in prevalence is, however, expected to occur in Asia and Africa, where most patients will probably be found by 2030.[31] The increase in incidence in developing countries follows the trend of urbanization and lifestyle changes, perhaps most importantly a "Western-style" diet. This has suggested an environmental (i.e., dietary) effect, but there is little understanding of the mechanism(s) at present, though there is much speculation, some of it most compellingly presented.[31]
United States
For at least 20 years, diabetes rates in North America have been increasing substantially. In 2010, nearly 26 million people have diabetes in the United States alone, from those 7 million people remain undiagnosed. Another 57 million people are estimated to have prediabetes.[32][33]
The Centers for Disease Control and Prevention (CDC) has termed the change an epidemic.[34] The National Diabetes Information Clearinghouse estimates diabetes costs $132 billion in the United States alone every year. About 5%–10% of diabetes cases in North America are type 1, with the rest being type 2. The fraction of type 1 in other parts of the world differs. Most of this difference is not currently understood. The American Diabetes Association (ADA) cites the 2003 assessment of the National Center for Chronic Disease Prevention and Health Promotion (Centers for Disease Control and Prevention) that one in three Americans born after 2000 will develop diabetes in their lifetimes.[35][36]
According to the ADA, about 18.3% (8.6 million) of Americans age 60 and older have diabetes.[37] Diabetes mellitus prevalence increases with age, and the numbers of older persons with diabetes are expected to grow as the elderly population increases in number. The National Health and Nutrition Examination Survey (NHANES III) demonstrated, in the population over 65 years old, 18% to 20% have diabetes, with 40% having either diabetes or its precursor form of impaired glucose tolerance.[38]
Australia
Indigenous populations in first world countries have a higher prevalence and increasing incidence of diabetes than their corresponding nonindigenous populations. In Australia, the age-standardised prevalence of self-reported diabetes in indigenous Australians is almost four times that of nonindigenous Australians.[39] Preventative community health programs, such as Sugar Man (diabetes education), are showing some success in tackling this problem.
United Kingdom
About 3.8 million people in the United Kingdom have diabetes mellitus, but the charity Diabetes U.K. have made predictions that that could become high as 6.2 million by 2035/2036. Diabetes U.K. have also predicted that the National Health Service could be spending as much as 16.9 billion pounds on diabetes mellitus by 2035, a figure that means that the National Health Service could be spending as much as 17% of its budget on diabetes treatment by 2035.[40]
History
Diabetes was one of the first diseases described,[41] with an Egyptian manuscript from c. 1500 BCE mentioning "too great emptying of the urine".[42] The first described cases are believed to be of type 1 diabetes.[42] Indian physicians around the same time identified the disease and classified it as madhumeha or "honey urine", noting the urine would attract ants.[42] The term "diabetes" or "to pass through" was first used in 230 BCE by the Greek Appollonius of Memphis.[42] The disease was rare during the time of the Roman empire, with Galen commenting he had only seen two cases during his career.[42] Type 1 and type 2 diabetes where identified as separate conditions for the first time by the Indian physicians Sushruta and Charaka in 400-500 AD with type 1 associated with youth and type 2 with being overweight.[42] The term "mellitus" or "from honey" was added by the Briton John Rolle in the late 1700s to separate the condition from diabetes insipidus, which is also associated with frequent urination.[42] While many measure where tried, effective treatment was not developed until the early part of the 20th century, when Canadians Frederick Banting and Charles Herbert Best developed insulin in 1921 and 1922.[42] This was followed by the development of the long-acting insulin NPH in the 1940s.[42]
Etymology
The word diabetes (/[invalid input: 'icon']ˌdaɪ.əˈbiːtiːz/ or /ˌdaɪ.əˈbiːt[invalid input: 'ɨ']s/) comes from Latin diabētēs, which in turn comes from Ancient Greek διαβήτης (diabētēs) which literally means "a passer through; a siphon."[43] Ancient Greek physician Aretaeus of Cappadocia (fl. 1st century CE) used that word, with the intended meaning "excessive discharge of urine", as the name for the disease.[44][45] Ultimately, the word comes from Greek διαβαίνειν (diabainein), meaning "to pass through,"[43] which is composed of δια- (dia-), meaning "through" and βαίνειν (bainein), meaning "to go".[44] The word "diabetes" is first recorded in English, in the form diabete, in a medical text written around 1425.
The word mellitus (/m[invalid input: 'ɨ']ˈlaɪtəs/ or /ˈmɛl[invalid input: 'ɨ']təs/) comes from the classical Latin word mellītus, meaning "mellite"[46] (i.e. sweetened with honey;[46] honey-sweet[47]). The Latin word comes from mell-, which comes from mel, meaning "honey";[46][47] sweetness;[47] pleasant thing,[47] and the suffix -ītus,[46] whose meaning is the same as that of the English suffix "-ite".[48] It was Thomas Willis who in 1675 added "mellitus" to the word "diabetes" as a designation for the disease, when he noticed the urine of a diabetic had a sweet taste (glycosuria).[45] This sweet taste had been noticed in urine by the ancient Greeks, Chinese, Egyptians, Indians, and Persians.
Society and culture
The 1990 "St. Vincent Declaration"[49][50] was the result of international efforts to improve the care accorded to those with diabetes. Doing so is important not only in terms of quality of life and life expectancy, but also economically—expenses due to diabetes have been shown to be a major drain on health- and productivity-related resources for healthcare systems and governments.
Several countries established more and less successful national diabetes programmes to improve treatment of the disease.[51]
Diabetic patients with neuropathic symptoms such as numbness or tingling in feet or hands are twice as likely to be unemployed as those without the symptoms.[52]
In other animals
In animals, diabetes is most commonly encountered in dogs and cats. Middle-aged animals are most commonly affected. Female dogs are twice as likely to be affected as males, while according to some sources, male cats are also more prone than females. In both species, all breeds may be affected, but some small dog breeds are particularly likely to develop diabetes, such as Miniature Poodles.[53] The symptoms may relate to fluid loss and polyuria, but the course may also be insidious. Diabetic animals are more prone to infections. The long-term complications recognised in humans are much rarer in animals. The principles of treatment (weight loss, oral antidiabetics, subcutaneous insulin) and management of emergencies (e.g. ketoacidosis) are similar to those in humans.[53]
References
- ^ "Diabetes Blue Circle Symbol". International Diabetes Federation. 17 March 2006.
- ^ a b c d Shoback, edited by David G. Gardner, Dolores (2011). Greenspan's basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. pp. Chapter 17. ISBN 0-07-162243-8.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b c Williams textbook of endocrinology (12th ed.). Philadelphia: Elsevier/Saunders. pp. 1371–1435. ISBN 978-1-4377-0324-5.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1383/medc.30.1.1.28264, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1383/medc.30.1.1.28264instead. - ^ Rother KI (2007). "Diabetes treatment—bridging the divide". The New England Journal of Medicine. 356 (15): 1499–501. doi:10.1056/NEJMp078030. PMID 17429082.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b "Diabetes Mellitus (DM): Diabetes Mellitus and Disorders of Carbohydrate Metabolism: Merck Manual Professional". Merck Publishing. 2010. Retrieved 2010-07-30.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Dorner M, Pinget M, Brogard JM (1977). "Essential labile diabetes". MMW Munch Med Wochenschr (in German). 119 (19): 671–4. PMID 406527.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lawrence JM, Contreras R, Chen W, Sacks DA (2008). "Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005". Diabetes Care. 31 (5): 899–904. doi:10.2337/dc07-2345. PMID 18223030.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Handelsman, Yehuda; MD. "A Doctor's Diagnosis: Prediabetes". Power of Prevention. 1 (2): 2009.
{{cite journal}}: Cite has empty unknown parameter:|author-name-separator=(help); Unknown parameter|author-separator=ignored (help) - ^ a b "Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications" (PDF). World Health Organisation. 1999.
- ^ Cooke DW, Plotnick L (2008). "Type 1 diabetes mellitus in pediatrics". Pediatr Rev. 29 (11): 374–84, quiz 385. doi:10.1542/pir.29-11-374. PMID 18977856.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Emerging Risk Factors Collaboration (2010). "Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies". The Lancet. 375 (9733): 2215–22. doi:10.1016/S0140-6736(10)60484-9.
- ^ Boussageon R; Bejan-Angoulvant T; Saadatian-Elahi M; et al. (2011). "Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials". BMJ. 343: d4169. doi:10.1136/bmj.d4169. PMC 3144314. PMID 21791495.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Risérus U, Willett WC, Hu FB (2009). "Dietary fats and prevention of type 2 diabetes". Progress in Lipid Research. 48 (1): 44–51. doi:10.1016/j.plipres.2008.10.002. PMC 2654180. PMID 19032965.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Unless otherwise specified, reference is: Table 20-5 in Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson. Robbins Basic Pathology. Philadelphia: Saunders. ISBN 1-4160-2973-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) 8th edition. - ^ Sattar, N (2010-02-27). "Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials". The Lancet. 375 (9716): 735–42. doi:10.1016/S0140-6736(09)61965-6. PMID 20167359.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation (PDF). Geneva: World Health Organization. 2006. p. 21. ISBN 978-92-4-159493-6.
- ^ Vijan S (March 2010). "In the clinic. Type 2 diabetes". Annals of Internal Medicine. 152 (5): ITC31-15, quiz ITC316. doi:10.7326/0003-4819-152-5-201003020-01003. PMID 20194231.
- ^ ""Diabetes Care" January 2010". American Diabetes Association. Retrieved 2010-01-29.
- ^ Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL (2001). "Postchallenge hyperglycemia and mortality in a national sample of U.S. adults". Diabetes Care. 24 (8): 1397–402. doi:10.2337/diacare.24.8.1397. PMID 11473076.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia : report of a WHO/IDF consultation (PDF). World Health Organization. 2006. p. 21. ISBN 978-92-4-159493-6.
- ^ Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, Booker L, Yazdi H. "Diagnosis, Prognosis, and Treatment of Impaired Glucose Tolerance and Impaired Fasting Glucose". Summary of Evidence Report/Technology Assessment, No. 128. Agency for Healthcare Research and Quality. Retrieved 2008-07-20.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Selvin E; Steffes MW; Zhu H; et al. (2010). "Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults" (PDF). N. Engl. J. Med. 362 (9): 800–11. doi:10.1056/NEJMoa0908359. PMC 2872990. PMID 20200384.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Nathan DM; Cleary PA; Backlund JY; et al. (2005). "Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes" (PDF). The New England Journal of Medicine. 353 (25): 2643–53. doi:10.1056/NEJMoa052187. PMC 2637991. PMID 16371630.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ <Please add first missing authors to populate metadata.> (1995). "The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group". Annals of Internal Medicine. 122 (8): 561–8. doi:10.1059/0003-4819-122-8-199504150-00001. PMID 7887548.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b National Institute for Health and Clinical Excellence. Clinical guideline 66: Type 2 diabetes. London, 2008.
- ^ Adler AI; Stratton IM; Neil HA; et al. (2000). "Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study". BMJ. 321 (7258): 412–9. doi:10.1136/bmj.321.7258.412. PMC 27455. PMID 10938049.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b c Ripsin, CM (2009-01-01). "Management of blood glucose in type 2 diabetes mellitus" (PDF). American family physician. 79 (1): 29–36. PMID 19145963.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pignone M; Alberts MJ; Colwell JA; et al. (2010). "Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation". Diabetes Care. 33 (6): 1395–402. doi:10.2337/dc10-0555. PMC 2875463. PMID 20508233.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K (2009). "Home telehealth for diabetes management: a systematic review and meta-analysis". Diabetes Obes Metab. 11 (10): 913–30. doi:10.1111/j.1463-1326.2009.01057.x. PMID 19531058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Wild, S; Roglic, G; Green, A; Sicree, R; King, H (2004). "Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030". Diabetes Care. 27 (5): 1047–53. doi:10.2337/diacare.27.5.1047. PMID 15111519.
- ^ "Number of Americans with Diabetes Rises to Nearly 26 Million" (Press release). Centers for Disease Control and Prevention. 2011-01-26. Retrieved 2012-05-27.
- ^ "National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011" (PDF). Centers for Disease Control and Prevention. 2011. Retrieved 2012-05-31.
- ^ "Diabetes Rates Rise Another 6 Percent in 1999 — January 26, 2001". Retrieved 2008-06-23.
- ^ Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF (2003). "Lifetime risk for diabetes mellitus in the United States". JAMA. 290 (14): 1884–90. doi:10.1001/jama.290.14.1884. PMID 14532317.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Total Prevalence of Diabetes & Pre-diabetes". American Diabetes Association. 2005. Archived from the original on 2006-02-08. Retrieved 2006-03-17.
- ^ "Seniors and Diabetes". Elderly And Diabetes-Diabetes and Seniors. LifeMed Media. 2006. Retrieved 2007-05-14.
- ^ Harris MI; Flegal KM; Cowie CC; et al. (1998). "Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994". Diabetes Care. 21 (4): 518–24. doi:10.2337/diacare.21.4.518. PMID 9571335.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Australian Institute for Health and Welfare. "Diabetes, an overview". Archived from the original on 2008-06-17. Retrieved 2008-06-23.
- ^ "NHS spending on diabetes 'to reach £16.9 billion by 2035'". 2012-04-25. Retrieved 2012-04-26.
- ^ Ripoll, Brian C. Leutholtz, Ignacio (2011-04-25). Exercise and disease management (2nd ed.). Boca Raton: CRC Press. p. 25. ISBN 978-1-4398-2759-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i editor, Leonid Poretsky, (2009). Principles of diabetes mellitus (2nd ed.). New York: Springer. p. 3. ISBN 978-0-387-09840-1.
{{cite book}}:|last=has generic name (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b Oxford English Dictionary. diabetes. Retrieved 2011-06-10.
- ^ a b Harper, Douglas (2001–2010). "Online Etymology Dictionary. diabetes.". Retrieved 2011-06-10.
- ^ a b Dallas, John (2011). "Royal College of Physicians of Edinburgh. Diabetes, Doctors and Dogs: An exhibition on Diabetes and Endocrinology by the College Library for the 43rd St. Andrew's Day Festival Symposium".
- ^ a b c d Oxford English Dictionary. mellite. Retrieved 2011-06-10.
- ^ a b c d "MyEtimology. mellitus.". Retrieved 2011-06-10.
- ^ Oxford English Dictionary. -ite. Retrieved 2011-06-10.
- ^ Theodore H. Tulchinsky, Elena A. Varavikova (2008). The New Public Health, Second Edition. New York: Academic Press. p. 200. ISBN 0-12-370890-7.
- ^ Piwernetz K, Home PD, Snorgaard O, Antsiferov M, Staehr-Johansen K, Krans M (1993). "Monitoring the targets of the St Vincent Declaration and the implementation of quality management in diabetes care: the DIABCARE initiative. The DIABCARE Monitoring Group of the St Vincent Declaration Steering Committee". Diabetic Medicine. 10 (4): 371–7. doi:10.1111/j.1464-5491.1993.tb00083.x. PMID 8508624.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dubois, HFW and Bankauskaite, V (2005). "Type 2 diabetes programmes in Europe" (PDF). Euro Observer. 7 (2): 5–6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA (2007). "Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce". J. Occup. Environ. Med. 49 (6): 672–9. doi:10.1097/JOM.0b013e318065b83a. PMID 17563611.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b "Diabetes mellitus". Merck Veterinary Manual, 9th edition (online version). 2005. Retrieved 2011-10-23.
External links
- Diabetes at the Open Directory Project
- American Diabetes Association
- IDF Diabetes Atlas
- National Diabetes Education Program
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
Template:Link FA Template:Link FA Template:Link FA Template:Link FA
