Glycolipid
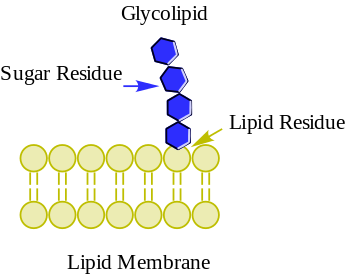
Glycolipids are lipids with a carbohydrate attached by a glycosidic bond.[1] Their role is to maintain stability of the membrane and to facilitate cellular recognition.[2] The carbohydrates are found on the outer surface of all eukaryotic cell membranes. They extend from the phospholipid bilayer into the aqueous environment outside the cell where it acts as a recognition site for specific chemicals as well as helping to maintain the stability of the membrane and attaching cells to one another to form tissues.[2]
Structure
The basic structure of a glycolipid is the presence of a carbohydrate monosaccharide or oligosaccharide bound to a lipid moiety. The lipid complex is most often composed of either a glycerol or sphingosine backbone, which gives rise to the two main categories of glycolipids, glyceroglycolipids and sphingolipids. Lipids are non-polar molecules, providing them the capability of interacting with the lipid-bilayer of the cell membrane and anchoring the glycolipid to the surface of the cell. Carbohydrates are used as the ligand component of glycolipids and their structure varies depending on the structure of the molecule it binds to. The carbohydrate contains polar groups that enable the molecule to be soluble in the aqueous environment surrounding the cell.[3] The two molecular groups form a glycoconjugate through a covalent bond referred to as a glycosidic bond. The anomeric carbon of the sugar binds to the hydroxyl group present on the lipid.
Metabolism
Glycosyltransferases
Glycolipids formation is dependent on the activity of glycosyltransferases. Glycoslytransferases are the enzymes responsible for catalyzing the reaction of the covalent bond formation linking the carbohydrate complex to the lipid molecule. It also functions to form the correct oligosaccharide that will be present in the complete structure to act as the receptor for cell signaling. The glycolipid is assembled in the golgi-apparatus and transported to the membrane via vesicles where the lipid remains embedded in the membrane and the carbohydrate is on the outer surface of the cell.[4]
Glycoside hydrolases
Glycoside hydrolases catalyze the breakage of glycosidic bonds. They are used to modify the glycan after it has been added onto the lipid to represent the final oligosaccharide structure. It is also involved in the natural degradation of glycolipids into their non-conjugated forms. The lipids and carbohydrates will then assume their common uses as energy in the body.[5]
Defects in Metabolism
Sphingolipidoses can be associated with defects in metabolism. Sphingolipidoses are a category of diseases that can be associated with the accumulation of sphingolipids from failure to degrade compounds correctly. Sphingolipidoses are typically inherited and have varying effects among the various types of diseases. One notable example is Niemann–Pick disease which can cause pain to, damage to neural networks, and, in severe cases, death.[6]
Function
cell–cell Interactions
The main function of glycolipids in the body is to serve as recognition sites for cell–cell interactions. The sugar moiety of the glycolipid will bind to a specific complementary carbohydrate or lectin, type of cell-surface protein, of a neighboring cell. The interaction of these cell surface markers initiates cellular responses that contribute to activities such as cell recognition, regulation, growth, and apoptosis.[7] Sphingolipidoses can be associated with defects in metabolism.
Immune Responses
An example of how glycolipids function within the body is the interaction between leukocytes and endothelial cells during inflammation. Selectins on the surface of leukocytes and endothelial cells will bind to the carbohydrates attached to glycolipids to initiate the immune response. This binding allows for leukocytes to leave circulation and congregate near the site of inflammation. This is the initial binding mechanism, after which it is followed by expression of integrins which form stronger bonds and allow leukocytes to migrate toward the specific site of inflammation.[8] Glycolipipds are also responsible for other immune responses, notably the recognition of viruses within the body.
Blood types
Blood type are an example of how glycolipids on cell membranes mediate cell interactions with the surrounding environment. There are four different blood types present in humans (A, B, AB, O) that are determined by the sugar moiety attached to a specific glycolipid on blood cells. Blood type A individuals have an N-acetylgalactosamine sugar attachment as the main determining structure, blood type B has a galactose, and Blood type O has no extra addition to the oligosaccharide. Erythroctes have the specific glycolipid for that individuals blood type covering their surface (with AB individuals have both types of antigens). Antibodies are produced in the body and bind to a specific blood type's glycolipids. Blood type A individuals produce antibodies that bind to the B antigen and so forth, so that any cells possessing the corresponding antigen will be targeted by the antibodies.[9]

Types of glycolipids
The following is an incomplete listing of glycolipid types.
- Glyceroglycolipids: Glyceroglycolipids are a sub-group of glycolipids characterized by an acetylated or non-acetylated glycerol with at least one fatty acid as the lipid complex. Glyceroglycolipids are often associated with photosynthetic membranes and the functions within them. Smaller sub-branches exist within this category of glycolipids depending upon the carbohydrate attached.[10]
- Galactolipids: Galactolipids are defined by a galactose sugar attached to glycerol lipid molecule. They are found in chloroplast membranes and are associated with photosynthetic properties.[10]
- Sulfolipids (SQDG): Sulfolipids possess a sulfur-containing functional group in the sugar moiety attached to a lipid and can be associated with the sulfur cycle in plants.[11]
- Glycosphingolipids: Glycosphingolipids are a sub-group of glycolipids characterized by a sphingosine as the lipid complex. A sphingosine is a long carbon chain with an amine and alcohol functional groups. Glycosphingolipids are mostly located in nervous tissue and are responsible for cell signaling.[12]
- Cerebrosides: Cerebrosides are a group glycosphingolipids involved in nerve cell membranes.[13]
- Galactocerebrosides: Galactocerebrosides are types of cerebroseides with a galactose group and specifically involved in neural tissue and other tissues respectively
- Glucocerebrosides: Glucocerebrosides are a classified as a glycolipid with a cerebroside lipid base with glucose as the carbohydrate head group and are often found in non-neural tissue.
- Sulfatides: Sulfatides are a class of glycolipids containing a sulfate group in the carbohydrate with a ceramide lipid backbone. They are involved in numerous biological functions ranging from immune response to nervous system signaling.
- Gangliosides: Gangliosides are the most complex animal glycolipids; contain negatively charged oligosacchrides with one or more sialic acid residues; more than 200[14] different gangliosides have been identified; they are most abundant in nerve cells)
- Globosides: Globosides are glycosphingolipids with more than one sugar as part of the carbohydrate complex. They have a variety of functions and failure of degradation of these molecules leads to Fabry's disease.
- Glycophosphosphingolipids: Glycophosphosphingolipid are complex glycophospholipids from fungi, including yeasts, and in plants, where they were originally called "phytoglycolipids" by Herbert Carter, et al., may comprise as complicated a set of compounds as the negatively charged gangliosides in animals. The head group of a glycolipid is composed of sugars.
- Cerebrosides: Cerebrosides are a group glycosphingolipids involved in nerve cell membranes.[13]
- Glycophosphatidylinositols: Glycophosphatidylinositols are a sub-group of glycolipids defined by a phosphatidylinositol lipid moiety bound to a carbohydrate complex. Glycosylphosphatidylinositols can be bound to the C-terminus of a protein and have various functions associated with the different proteins they can be bound to.
See also
References
- ^ Voet, Donald; Voet, Judith; Pratt, Charlotte (2013). Fundamentals of Biochemistry Life at the Molecular Level (Fourth ed.). Hoboken, NJ: John Wiley & Sons, Inc. ISBN 9781118129180.
- ^ a b "Glycolipids". nature. Nature Publishing Group. Retrieved November 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Aureli, Massimo; et al. (August 2015). "Lipid Membrane domains in the Brain". BBA Molecular and Cell Biology of Lipids. 1851 (8): 1006–1016. doi:10.1016/j.bbalip.2015.02.001. PMID 25677824.
- ^ Williams, GJ; Thorson, JS (2009). "Natural product glycosyltransferases: properties and applications". Advances in Enzymology and Related Areas of Molecular Biology. 76: 55–119. doi:10.1002/9780470392881.ch2. PMID 18990828.
- ^ Sinnott, M. L. "Catalytic mechanisms of enzymatic glycosyl transfer". Chem. Rev. 1990, 90, 1171–1202.
- ^ Sandhoff, Kondrad (December 2015). "Sphingolipidoses". Journal of Clinical Pathology. 68 (12): 94–105. doi:10.1136/jcp.s3-8.1.94.
- ^ Schnaar, Ronald (15 June 2004). "Glycolipid-mediated cell–cell recognition in inflammation and nerve regeneration". Archives of Biochemistry and Biophysics. 426 (2): 163–172. doi:10.1016/j.abb.2004.02.019. PMID 15158667.
- ^ Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. cell–cell Interactions. Available from: http://www.ncbi.nlm.nih.gov/books/NBK9851/
- ^ Erb IH (1 May 1940). "Blood Group Classifications, a Plea for Uniformity". Canadian Medical Association Journal. 42 (5): 418–21. PMC 537907. PMID 20321693.
- ^ a b Neufeld, Elizabeth (January 1964). "Formation of galactolipids". Biochemical and Biophysical Research. 14 (6): 503–508. doi:10.1016/0006-291X(64)90259-1.
- ^ Harwood, John L.; Nicholls, Rodney G. (1979). "The plant sulfolipid. A major component of the sulfur cycle". Biochemical Society Transactions. 7 (2): 440–7. doi:10.1042/bst0070440. PMID 428677.
- ^ Hakomori, S (December 1995). "Functional role of glycosphingolipids in cell recognition and signaling". The Journal of Biochemistry. 118 (6): 1091–1103. PMID 8720120.
- ^ Jurevics, H; Hostettler, J; Muse, ED; Sammond, DW; Matsushima, GK; Toews, AD; Morell, P (May 2001). "Cerebroside synthesis as a measure of the rate of remyelination following cuprizone-induced demyelination in brain". Journal of Neurochemistry. 77 (4): 1067–76. doi:10.1046/j.1471-4159.2001.00310.x. PMID 11359872.
- ^ Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease – a review-JOURNAL OF LIPID RESEARCH Volume: 49 Issue: 6 Pages: 1157–1175 Published: JUN 2008
- ^ Paulick, Margot G.; Bertozzi, Carolyn R. (8 July 2008). "The Glycosylphosphatidylinositol Anchor: A Complex Membrane-Anchoring Structure for Proteins". Biochemistry. 47 (27): 6991–7000. doi:10.1021/bi8006324. ISSN 0006-2960. PMC 2663890. PMID 18557633.
Genetic Science Learning Center. "Genes and Blood Type." Learn.Genetics 24 November 2015 <http://learn.genetics.utah.edu/content/inheritance/blood/>
External links
- Glycolipids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
