Mycobacterium tuberculosis
| Mycobacterium tuberculosis | |
|---|---|

| |
| M. tuberculosis bacterial colonies | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Suborder: | |
| Family: | |
| Genus: | |
| Species: | M. tuberculosis
|
| Binomial name | |
| Mycobacterium tuberculosis Zopf 1883
| |
| Synonyms | |
|
Tubercle bacillus Koch 1882 | |
Mycobacterium tuberculosis (MTB) is a pathogenic bacterial species in the genus Mycobacterium and the causative agent of most cases of tuberculosis (TB).[1] First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface (primarily mycolic acid), which makes the cells impervious to Gram staining, so acid-fast detection techniques are used, instead. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, MTB infects the lungs. The most frequently used diagnostic methods for TB are the tuberculin skin test, acid-fast stain, and chest radiographs.[1]
The M. tuberculosis genome was sequenced in 1998.[2][3]
Pathophysiology
M. tuberculosis requires oxygen to grow. It does not retain any bacteriological stain due to high lipid content in its wall, and thus is neither Gram-positive nor Gram-negative; hence Ziehl-Neelsen staining, or acid-fast staining, is used. While mycobacteria do not seem to fit the Gram-positive category from an empirical standpoint (i.e., they do not retain the crystal violet stain), they are classified as acid-fast Gram-positive bacteria due to their lack of an outer cell membrane.[1]
M. tuberculosis divides every 15–20 hours, which is extremely slow compared to other bacteria, which tend to have division times measured in minutes (Escherichia coli can divide roughly every 20 minutes). It is a small bacillus that can withstand weak disinfectants and can survive in a dry state for weeks. Its unusual cell wall, rich in lipids (e.g., mycolic acid), is likely responsible for this resistance and is a key virulence factor.[4]
When in the lungs it is bad, M. tuberculosis is taken up by alveolar macrophages, but they are unable to digest the bacterium. Its cell wall prevents the fusion of the phagosome with a lysosome. Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1); however, this blockade does not prevent fusion of vesicles filled with nutrients. Consequently, the bacteria multiply unchecked within the macrophage. The bacteria also carried the UreC gene, which prevents acidification of the phagosome.[5] The bacteria also evade macrophage-killing by neutralizing reactive nitrogen intermediates.[6]
The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced our understanding of the pathogenesis and virulence factors of M. tuberculosis. Many secreted and exported proteins are known to be important in pathogenesis.[7]
Strain variation
M. tuberculosis comes from the genus Mycobacterium, which is composed of approximately 100 recognized and proposed species. The most familiar of the species are M. tuberculosis and M. leprae (leprosy).[8]
M. tuberculosis is genetically diverse, which results in significant phenotypic differences between clinical isolates. Different strains of M. tuberculosis are associated with different geographic regions. However, phenotypic studies suggest that strain variation never has implications for the development of new diagnostics and vaccines. Microevolutionary variation does affect the relative fitness and transmission dynamics of antibiotic-resistant strains.[9]
Typing of strains is useful in the investigation of tuberculosis outbreaks, because it gives the investigator evidence for-or-against transmission from person to person. Consider the situation where person A has tuberculosis and believes that he acquired it from person B. If the bacteria isolated from each person belong to different types, then transmission from B to A is definitively disproved; on the other hand, if the bacteria are the same strain, then this supports (but does not definitively prove) the theory that B infected A.
Until the early 2000s, M. tuberculosis strains were typed by pulsed field gel electrophoresis (PFGE).[10][11] This has now been superseded by variable numbers of tandem repeats (VNTR), which is technically easier to perform and allows better discrimination between strains. This method makes use of the presence of repeated DNA sequences within the M. tuberculosis genome.
There are three generations of VNTR typing for M. tuberculosis. The first scheme, called ETR (exact tandem repeat), used only five loci,[12] but the resolution afforded by these five loci was not as good as PFGE. The second scheme, called MIRU (mycobacterial interspersed repetitive unit) had discrimination as good as PFGE.[13][14] The third generation (MIRU2) added a further nine loci to bring the total to 24. This provides a degree of resolution greater than PFGE and is currently the standard for typing M. tuberculosis.[15]
Hypervirulent strains
Mycobacterium outbreaks are often caused by hypervirulent strains of M. tuberculosis. In laboratory experiments, these clinical isolates elicit unusual immunopathology, and may be either hyperinflammatory or hypoinflammatory. Studies have shown the majority of hypervirulent mutants have deletions in their cell wall-modifying enzymes or regulators that respond to environmental stimuli. Studies of these mutants have indicated the mechanisms that enable M. tuberculosis to mask its full pathogenic potential, inducing a granuloma that provides a protective niche, and enable the bacilli to sustain a long-term, persistent infection.[16]
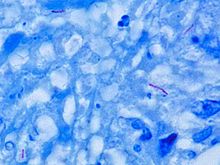
Microscopy
M. tuberculosis is characterized by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei. Organisms are identified by their red color on acid-fast staining.
Genome
The genome of the H37Rv strain was published in 1998.[17] Its size is 4 million base pairs, with 3959 genes; 40% of these genes have had their function characterised, with possible function postulated for another 44%. Within the genome are also six pseudogenes.
The genome contains 250 genes involved in fatty acid metabolism, with 39 of these involved in the polyketide metabolism generating the waxy coat. Such large numbers of conserved genes show the evolutionary importance of the waxy coat to pathogen survival.
About 10% of the coding capacity is taken up by two clustered gene families that encode acidic, glycine-rich proteins. These proteins have a conserved N-terminal motif, deletion of which impairs growth in macrophages and granulomas.[18]
Nine noncoding sRNAs have been characterised in M. tuberculosis,[19] with a further 56 predicted in a bioinformatics screen.[20]
History
M. tuberculosis, then known as the "tubercle bacillus", was first described on 24 March 1882 by Robert Koch, who subsequently received the Nobel Prize in physiology or medicine for this discovery in 1905; the bacterium is also known as "Koch's bacillus".[21]
Tuberculosis has existed throughout history, but the name has changed frequently over time. In 1720, though, the history of tuberculosis started to take shape into what is known of it today; as the physician Benjamin Marten described in his A Theory of Consumption, tuberculosis may be caused by small living creatures that are transmitted through the air to other patients.[22]
See also
References
- ^ a b c Ismael Kassim, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
{{cite book}}:|author=has generic name (help) - ^ Cole ST, Brosch R, Parkhill J; et al. (1998). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature. 393 (6685): 537–44. doi:10.1038/31159. PMID 9634230.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Camus JC, Pryor MJ, Médigue C, Cole ST (2002). "Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv". Microbiology (Reading, Engl.). 148 (Pt 10): 2967–73. PMID 12368430.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Murray PR, Rosenthal KS, Pfaller MA (2005). Medical Microbiology. Elsevier Mosby.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bell E (2005). "Vaccines: A souped-up version of BCG". Nature Reviews Immunology. 5 (10): 746. doi:10.1038/nri1720.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ JoAnne L Flynn� and John Chany (2003). "Immune evasion by Mycobacterium tuberculosis: living with the enemy". Current Opinion in Immunology. 15 (4): 450.
{{cite journal}}: Unknown parameter|month=ignored (help); replacement character in|author=at position 15 (help) - ^ Wooldridge K (editor) (2009). Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press. ISBN 978-1-904455-42-4.
{{cite book}}:|author=has generic name (help) - ^ (Page 576;Textbook of Diagnostic Microbiology, Mahon, Lehman, Manuselis)
- ^ Gagneux S (2009). "Strain variation and evolution". In Parish T, Brown A (ed.). Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ Y Zhang, G H Mazurek, M D Cave; et al. (1992). "DNA polymorphisms in strains of Mycobacterium tuberculosis analyzed by pulsed-field gel electrophoresis: a tool for epidemiology". J Clin Microbiol. 30 (6): 1551–1556.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Singh SP, Salamon H, Lahti CJ, Farid-Moyer M, Small PM. (1999). "Use of pulsed-field gel electrophoresis for molecular epidemiologic and population genetic studies of Mycobacterium tuberculosis". J Clin Microbiol. 37 (6): 1927–31. PMID 10325348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frothingham R, Meeker-O'Connell WA. (1998). "Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats". Microbiology. 144 (Pt 5): 1189–96. PMID 9611793.
- ^ Mazars E, Lesjean S, Banuls AL; et al. (2001). "High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology". Proc Natl Acad Sci U S A. 98 (4): 1901–6. PMID 11172048.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Hawkey PM, Smith EG, Evans JT; et al. (2003). "Mycobacterial interspersed repetitive unit typing of Mycobacterium tuberculosis compared to IS6110-based restriction fragment length polymorphism analysis for investigation of apparently clustered cases of tuberculosis". J Clin Microbiol. 41 (8): 3514–20. PMID 12904348.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Supply P, Allix C, Lesjean S; et al. (2006). "Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis". J Clin Microbiol. 44 (12): 4498–510. PMID 17005759.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Casali N (2009). "Hypervirulent Mycobacterium tuberculosis". Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ "Mycobacterium tuberculosis". Sanger Institute. 29 March 2007. Retrieved 16 November 2008.
- ^ Glickman MS, Jacobs WR (2001). "Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline". Cell. 104 (4): 477–85. doi:10.1016/S0092-8674(01)00236-7. PMID 11239406.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Arnvig KB, Young DB (2009). "Identification of small RNAs in Mycobacterium tuberculosis". Mol. Microbiol. 73 (3): 397–408. doi:10.1111/j.1365-2958.2009.06777.x. PMC 2764107. PMID 19555452. Retrieved 31 August 2010.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Livny J, Brencic A, Lory S, Waldor MK (2006). "Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2". Nucleic Acids Res. 34 (12): 3484–93. doi:10.1093/nar/gkl453. PMC 1524904. PMID 16870723. Retrieved 31 August 2010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) [dead link] - ^ "Robert Koch and Tuberculosis: Koch's Famous Lecture". Nobel Foundation. 2008. Retrieved 18 November 2008.
- ^ "Tuberculosis History Timeline". Retrieved 18 June 2010.
External links
TB database: an integrated platform for Tuberculosis research
