Mycobacterium tuberculosis
| Mycobacterium tuberculosis | |
|---|---|

| |
| M. tuberculosis colonies | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Mycobacteriales |
| Family: | Mycobacteriaceae |
| Genus: | Mycobacterium |
| Species: | M. tuberculosis
|
| Binomial name | |
| Mycobacterium tuberculosis Zopf 1883
| |
| Synonyms | |
|
Tubercle bacillus Koch 1882 | |
This article needs to be updated. (December 2022) |
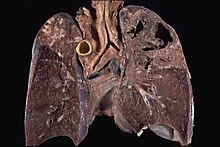
Mycobacterium tuberculosis (M. tb), also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis.[1][2] First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear weakly Gram-positive.[3] Acid-fast stains such as Ziehl–Neelsen, or fluorescent stains such as auramine are used instead to identify M. tuberculosis with a microscope. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.[2][4]
The M. tuberculosis genome was sequenced in 1998.[5][6]
Microbiology[edit]
In 2019, M. tuberculosis was found in a genetically related complex group of Mycobacterium species called Mycobacterium tuberculosis complex that has at least 9 members:
- M. tuberculosis[7] sensu stricto
- M. africanum[7]
- M. canettii[7]
- M. bovis[7]
- M. caprae[7]
- M. microti[7]
- M. pinnipedii[7]
- M. mungi[7]
- M. orygis[7]
It requires oxygen to grow, and is nonmotile.[8][9] M. tuberculosis divides every 18–24 hours. This is extremely slow compared with other bacteria, which tend to have division times measured in minutes (Escherichia coli can divide roughly every 20 minutes). It is a small bacillus that can withstand weak disinfectants and can survive in a dry state for weeks. Its unusual cell wall, rich in lipids such as mycolic acid and cord factor glycolipid, is likely responsible for its resistance to desiccation and is a key virulence factor.[10][11]
Microscopy[edit]
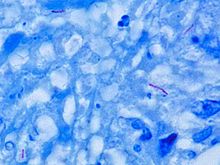
Other bacteria are commonly identified with a microscope by staining them with Gram stain. However, the mycolic acid in the cell wall of M. tuberculosis does not absorb the stain. Instead, acid-fast stains such as Ziehl–Neelsen stain, or fluorescent stains such as auramine are used.[4] Cells are curved rod-shaped and are often seen wrapped together, due to the presence of fatty acids in the cell wall that stick together.[12] This appearance is referred to as cording, like strands of cord that make up a rope.[9] M. tuberculosis is characterized in tissue by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei.
Culture[edit]

- Negative control
- M. tuberculosis: Dry-appearing colonies
- Mycobacterium avium complex: Wet-appearing colonies
- M. gordonae: Yellowish colonies

M. tuberculosis can be grown in the laboratory. Compared to other commonly studied bacteria, M. tuberculosis has a remarkably slow growth rate, doubling roughly once per day. Commonly used media include liquids such as Middlebrook 7H9 or 7H12, egg-based solid media such as Lowenstein-Jensen, and solid agar-based such as Middlebrook 7H11 or 7H10.[9] Visible colonies require several weeks to grow on agar plates. Mycobacteria growth indicator tubes can contain a gel that emits fluorescent light if mycobacteria are grown. It is distinguished from other mycobacteria by its production of catalase and niacin.[13] Other tests to confirm its identity include gene probes and MALDI-TOF.[14][15]
Morphology[edit]
Analysis of Mycobacterium tuberculosis via scanning electron microscope shows the bacteria are 2.71 ± 1.05μm in length with an average diameter of 0.345 ± 0.029 μm.[16] The outer membrane and plasma membrane surface areas were measured to be 3.04 ± 1.33 μm2 and 2.67 ± 1.19 μm2, respectively. The cell, outer membrane, periplasm, plasma membrane, and cytoplasm volumes were 0.293 ± 0.113 fl (= μm3), 0.006 ± 0.003 fl, 0.060 ± 0.021 fl, 0.019 ± 0.008 fl, and 0.210 ± 0.091 fl, respectively. The average total ribosome number was 1,672 ± 568 with ribosome density about 716.5 ± 171.4/0.1 fl.[16]
| Feature | Magnitude |
|---|---|
| Length | 2.71 ± 1.05μm |
| Outer membrane surface area | 3.04 ± 1.33 μm2 |
| Cell volume | 0.293 ± 0.113 fl (= μm3) |
Pathophysiology[edit]
Humans are the only known reservoirs of M. tuberculosis. A misconception is that M. tuberculosis can be spread by shaking hands, making contact with toilet seats, sharing food or drink, or sharing toothbrushes. However, major spread is through air droplets originating from a person who has the disease either coughing, sneezing, speaking, or singing.[17]
When in the lungs, M. tuberculosis is phagocytosed by alveolar macrophages, but they are unable to kill and digest the bacterium. Its cell wall is made of cord factor glycolipids that inhibit the fusion of the phagosome with the lysosome, which contains a host of antibacterial factors.[18][11]
Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1); however, this blockade does not prevent fusion of vesicles filled with nutrients. In addition, production of the diterpene isotuberculosinol prevents maturation of the phagosome.[19] The bacteria also evades macrophage-killing by neutralizing reactive nitrogen intermediates.[20] More recently, M. tuberculosis has been shown to secrete and cover itself in 1-tuberculosinyladenosine (1-TbAd), a special nucleoside that acts as an antacid, allowing it to neutralize pH and induce swelling in lysosomes.[21][22]
In M. tuberculosis infections, PPM1A levels were found to be upregulated, and this, in turn, would impact the normal apoptotic response of macrophages to clear pathogens, as PPM1A is involved in the intrinsic and extrinsic apoptotic pathways. Hence, when PPM1A levels were increased, the expression of it inhibits the two apoptotic pathways.[23] With kinome analysis, the JNK/AP-1 signalling pathway was found to be a downstream effector that PPM1A has a part to play in, and the apoptotic pathway in macrophages are controlled in this manner.[23] As a result of having apoptosis being suppressed, it provides M. tuberculosis with a safe replicative niche, and so the bacteria are able to maintain a latent state for a prolonged time.[24]
Granulomas, organized aggregates of immune cells, are a hallmark feature of tuberculosis infection. Granulomas play dual roles during infection: they regulate the immune response and minimize tissue damage, but also can aid in the expansion of infection.[25][26][27][28][29]
The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced the understanding of its pathogenesis and virulence factors. Many secreted and exported proteins are known to be important in pathogenesis.[30] For example, one such virulence factor is cord factor (trehalose dimycolate), which serves to increase survival within its host. Resistant strains of M. tuberculosis have developed resistance to more than one TB drug, due to mutations in their genes. In addition, pre-existing first-line TB drugs such as rifampicin and streptomycin have decreased efficiency in clearing intracellular M. tuberculosis due to their inability to effectively penetrate the macrophage niche.[31]
JNK plays a key role in the control of apoptotic pathways—intrinsic and extrinsic. In addition, it is also found to be a substrate of PPM1A activity,[32] hence the phosphorylation of JNK would cause apoptosis to occur.[33] Since PPM1A levels are elevated during M. tuberculosis infections, by inhibiting the PPM1A signalling pathways, it could potentially be a therapeutic method to kill M. tuberculosis-infected macrophages by restoring its normal apoptotic function in defence of pathogens.[23] By targeting the PPM1A-JNK signalling axis pathway, then, it could eliminate M. tuberculosis-infected macrophages.[23]
The ability to restore macrophage apoptosis to M. tuberculosis-infected ones could improve the current tuberculosis chemotherapy treatment, as TB drugs can gain better access to the bacteria in the niche.[34] thus decreasing the treatment times for M. tuberculosis infections.
Symptoms of M. tuberculosis include coughing that lasts for more than three weeks, hemoptysis, chest pain when breathing or coughing, weight loss, fatigue, fever, night sweats, chills, and loss of appetite. M. tuberculosis also has the potential of spreading to other parts of the body. This can cause blood in urine if the kidneys are affected, and back pain if the spine is affected.[35]
Strain variation[edit]
Typing of strains is useful in the investigation of tuberculosis outbreaks, because it gives the investigator evidence for or against transmission from person to person. Consider the situation where person A has tuberculosis and believes he acquired it from person B. If the bacteria isolated from each person belong to different types, then transmission from B to A is definitively disproven; however, if the bacteria are the same strain, then this supports (but does not definitively prove) the hypothesis that B infected A.
Until the early 2000s, M. tuberculosis strains were typed by pulsed field gel electrophoresis.[36] This has now been superseded by variable numbers of tandem repeats (VNTR), which is technically easier to perform and allows better discrimination between strains. This method makes use of the presence of repeated DNA sequences within the M. tuberculosis genome.
Three generations of VNTR typing for M. tuberculosis are noted. The first scheme, called exact tandem repeat, used only five loci,[37] but the resolution afforded by these five loci was not as good as PFGE. The second scheme, called mycobacterial interspersed repetitive unit, had discrimination as good as PFGE.[38][39] The third generation (mycobacterial interspersed repetitive unit – 2) added a further nine loci to bring the total to 24. This provides a degree of resolution greater than PFGE and is currently the standard for typing M. tuberculosis.[40] However, with regard to archaeological remains, additional evidence may be required because of possible contamination from related soil bacteria.[41]
Antibiotic resistance in M. tuberculosis typically occurs due to either the accumulation of mutations in the genes targeted by the antibiotic or a change in titration of the drug.[42] M. tuberculosis is considered to be multidrug-resistant (MDR TB) if it has developed drug resistance to both rifampicin and isoniazid, which are the most important antibiotics used in treatment. Additionally, extensively drug-resistant M. tuberculosis (XDR TB) is characterized by resistance to both isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).[43]

Genome[edit]
The genome of the H37Rv strain was published in 1998.[44][45] Its size is 4 million base pairs, with 3,959 genes; 40% of these genes have had their function characterized, with possible function postulated for another 44%. Within the genome are also six pseudogenes.
Fatty acid metabolism. The genome contains 250 genes involved in fatty acid metabolism, with 39 of these involved in the polyketide metabolism generating the waxy coat. Such large numbers of conserved genes show the evolutionary importance of the waxy coat to pathogen survival. Furthermore, experimental studies have since validated the importance of a lipid metabolism for M. tuberculosis, consisting entirely of host-derived lipids such as fats and cholesterol. Bacteria isolated from the lungs of infected mice were shown to preferentially use fatty acids over carbohydrate substrates.[46] M. tuberculosis can also grow on the lipid cholesterol as a sole source of carbon, and genes involved in the cholesterol use pathway(s) have been validated as important during various stages of the infection lifecycle of M. tuberculosis, especially during the chronic phase of infection when other nutrients are likely not available.[47]
PE/PPE gene families. About 10% of the coding capacity is taken up by the PE/PPE gene families that encode acidic, glycine-rich proteins. These proteins have a conserved N-terminal motif, deletion of which impairs growth in macrophages and granulomas.[48]
Noncoding RNAs. Nine noncoding sRNAs have been characterised in M. tuberculosis,[49] with a further 56 predicted in a bioinformatics screen.[50]
Antibiotic resistance genes. In 2013, a study on the genome of several sensitive, ultraresistant, and multiresistant M. tuberculosis strains was made to study antibiotic resistance mechanisms. Results reveal new relationships and drug resistance genes not previously associated and suggest some genes and intergenic regions associated with drug resistance may be involved in the resistance to more than one drug. Noteworthy is the role of the intergenic regions in the development of this resistance, and most of the genes proposed in this study to be responsible for drug resistance have an essential role in the development of M. tuberculosis.[51]
Epigenome. Single-molecule real-time sequencing and subsequent bioinformatic analysis has identified three DNA methyltransferases in M. tuberculosis, Mycobacterial Adenine Methyltransferases A (MamA),[52] B (MamB),[53] and C (MamC).[54] All three are adenine methyltransferases, and each are functional in some clinical strains of M. tuberculosisand not in others.[55][54] Unlike DNA methyltransferases in most bacteria, which invariably methylate the adenines at their targeted sequence,[56] some strains of M. tuberculosis carry mutations in MamA that cause partial methylation of targeted adenine bases.[54] This occurs as intracellular stochastic methylation, where a some targeted adenine bases on a given DNA molecule are methylated while others remain unmethylated.[54][57] MamA mutations causing intercellular mosaic methylation are most common in the globally successful Beijing sublineage of M. tuberculosis.[54] Due to the influence of methylation on gene expression at some locations in the genome[52], it has been hypothesized that IMM may give rise to phenotypic diversity, and partially responsible for the global success of Beijing sublineage.[54]
Evolution[edit]
The M. tuberculosis complex evolved in Africa and most probably in the Horn of Africa.[58][59] In addition to M. tuberculosis, the M. tuberculosis complex (MTBC) has a number of members infecting various animal species, these include M. africanum, M. bovis (Dassie's bacillus), M. caprae, M. microti, M. mungi, M. orygis, and M. pinnipedii. This group may also include the M. canettii clade. These animal strains of MTBC do not strictly deserve species status, as they are all closely related and embedded in the M. tuberculosis phylogeny, but for historic reasons, they currently hold species status.
The M. canettii clade – which includes M. prototuberculosis – is a group of smooth-colony Mycobacterium species. Unlike the established members of the M. tuberculosis group, they undergo recombination with other species. The majority of the known strains of this group have been isolated from the Horn of Africa. The ancestor of M. tuberculosis appears to be M. canettii, first described in 1969.[60]
The established members of the M. tuberculosis complex are all clonal in their spread. The main human-infecting species have been classified into seven lineages. Translating these lineages into the terminology used for spoligotyping, a very crude genotyping methodology, lineage 1 contains the East African-Indian (EAI), the Manila family of strains and some Manu (Indian) strains; lineage 2 is the Beijing group; lineage 3 includes the Central Asian (CAS) strains; lineage 4 includes the Ghana and Haarlem (H/T), Latin America-Mediterranean (LAM) and X strains; types 5 and 6 correspond to M. africanum and are observed predominantly and at high frequencies in West Africa. A seventh type has been isolated from the Horn of Africa.[58] The other species of this complex belong to a number of spoligotypes and do not normally infect humans.
Lineages 2, 3 and 4 all share a unique deletion event (tbD1) and thus form a monophyletic group.[61] Types 5 and 6 are closely related to the animal strains of MTBC, which do not normally infect humans. Lineage 3 has been divided into two clades: CAS-Kili (found in Tanzania) and CAS-Delhi (found in India and Saudi Arabia).
Lineage 4 is also known as the Euro-American lineage. Subtypes within this type include Latin American Mediterranean, Uganda I, Uganda II, Haarlem, X, and Congo.[62]
A much cited study reported that M. tuberculosis has co-evolved with human populations, and that the most recent common ancestor of the M. tuberculosis complex evolved between 40,000 and 70,000 years ago.[63][61] However, a later study that included genome sequences from M. tuberculosis complex members extracted from three 1,000-year-old Peruvian mummies, came to quite different conclusions. If the most recent common ancestor of the M. tuberculosis complex were 40,000 to 70,000 years old, this would necessitate an evolutionary rate much lower than any estimates produced by genomic analyses of heterochronous samples, suggesting a far more recent common ancestor of the M. tuberculosis complex as little as 6000 years ago.[64][65]
An analysis of over 3000 strains of M. bovis from 35 countries suggested an Africa origin for this species.[66]
Co-evolution with modern humans[edit]
There are currently two narratives existing in parallel regarding the age of MTBC and how it has spread and co-evolved with humans through time. One study compared the M. tuberculosis phylogeny to a human mitochondrial genome phylogeny and interpreted these as being highly similar. Based on this, the study suggested that M. tuberculosis, like humans, evolved in Africa and subsequently spread with anatomically modern humans out of Africa across the world. By calibrating the mutation rate of M. tuberculosis to match this narrative, the study suggested that MTBC evolved 40,000–70,000 years ago.[59] Applying this time scale, the study found that the M. tuberculosis effective population size expanded during the Neolithic Demographic Transition (around 10,000 years ago) and suggested that M. tuberculosis was able to adapt to changing human populations and that the historical success of this pathogen was driven at least in part by dramatic increases in human host population density. It has also been demonstrated that after emigrating from one continent to another, a human host's region of origin is predictive of which TB lineage they carry,[67][68] which could reflect either a stable association between host populations and specific M. tuberculosis lineages and/or social interactions that are shaped by shared cultural and geographic histories.
Regarding the congruence between human and M. tuberculosis phylogenies, a study relying on M. tuberculosis and human Y chromosome DNA sequences to formally assess the correlation between them, concluded that they are not congruent.[69] Also, a more recent study which included genome sequences from M. tuberculosis complex members extracted from three 1,000-year-old Peruvian mummies, estimated that the most recent common ancestor of the M. tuberculosis complex lived only 4,000 – 6,000 years ago.[70] The M. tuberculosis evolutionary rate estimated by the Bos et al. study[70] is also supported by a study on Lineage 4 relying on genomic aDNA sequences from Hungarian mummies more than 200 years old.[71] In total, the evidence thus favors this more recent estimate of the age of the MTBC most recent common ancestor, and thus that the global evolution and dispersal of M. tuberculosis has occurred over the last 4,000–6,000 years.
Among the seven recognized lineages of M. tuberculosis, only two are truly global in their distribution: Lineages 2 and 4. Among these, Lineage 4 is the most well dispersed, and almost totally dominates in the Americas. Lineage 4 was shown to have evolved in or in the vicinity of Europe, and to have spread globally with Europeans starting around the 13th century.[72] This study also found that Lineage 4 tuberculosis spread to the Americas shortly after the European discovery of the continent in 1492, and suggests that this represented the first introduction of human TB on the continent (although animal strains have been found in human remains predating Columbus.[70] Similarly, Lineage 4 was found to have spread from Europe to Africa during the Age of Discovery, starting in the early 15th century.[72]
It has been suggested that ancestral mycobacteria may have infected early hominids in East Africa as early as three million years ago.[73]
DNA fragments from M. tuberculosis and tuberculosis disease indications were present in human bodies dating from 7000 BC found at Atlit-Yam in the Levant.[74]
Antibiotic resistance (ABR)[edit]
M. tuberculosis is a clonal organism and does not exchange DNA via horizontal gene transfer. Despite an additionally slow evolution rate, the emergence and spread of antibiotic resistance in M. tuberculosis poses an increasing threat to global public health.[75] In 2019, the WHO reported the estimated incidence of antibiotic resistant TB to be 3.4% in new cases, and 18% in previously treated cases.[76] Geographical discrepancies exist in the incidence rates of drug-resistant TB. Countries facing the highest rates of ABR TB China, India, Russia, and South Africa.[76] Recent trends reveal an increase in drug-resistant cases in a number of regions, with Papua New Guinea, Singapore, and Australia undergoing significant increases.[77]
Multidrug-resistant Tuberculosis (MDR-TB) is characterised by resistance to at least the two front-line drugs isoniazid and rifampin.[76] MDR is associated with a relatively poor treatment success rate of 52%. Isoniazid and rifampin resistance are tightly linked, with 78% of the reported rifampin-resistant TB cases in 2019 being resistant to isoniazid as well.[76] Rifampin-resistance is primarily due to resistance-conferring mutations in the rifampin-resistance determining region (RRDR) within the rpoB gene.[78] The most frequently observed mutations of the codons in RRDR are 531, 526 and 516. However, alternative more elusive resistance-conferring mutations have been detected. Isoniazid function occurs through the inhibition of mycolic acid synthesis through the NADH-dependent enoyl-acyl carrier protein (ACP)-reductase.[79] This is encoded by the inhA gene. As a result, isoniazid resistance is primarily due to mutations within inhA and the KatG gene or its promoter region - a catalase peroxidase which is required to activate Isoniazid.[79] As MDR in M. tuberculosis becomes increasingly common, the emergence of pre-extensively drug resistant (pre-XDR) and extensively drug resistant (XDR-) TB threatens to exacerbate the public health crisis. XDR-TB is characterised by resistance to both rifampin and Isoniazid, as well second-line fluoroquinolones and at least one additional front-line drug.[76] Thus, the development of alternative therapeutic measures is of utmost priority.
An intrinsic contributor to the antibiotic resistant nature of M. tuberculosis is its unique cell wall. Saturated with long-chain fatty acids or mycolic acids, the mycobacterial cell presents a robust, relatively insoluble barrier.[80] This has led to its synthesis being the target of many antibiotics - such as Isoniazid. However, resistance has emerged to the majority of them. A novel, promising therapeutic target is mycobacterial membrane protein large 3 (MmpL3).[81] The mycobacterial membrane protein large (MmpL) proteins are transmembrane proteins which play a key role in the synthesis of the cell wall and the transport of the associated lipids. Of these, MmpL3 is essential; knock-out of which has been shown to be bactericidal.[81] Due to its essential nature, MmpL3 inhibitors show promise as alternative therapeutic measures in the age of antibiotic resistance. Inhibition of MmpL3 function showed an inability to transport trehalose monomycolate - an essential cell wall lipid - across the plasma membrane.[81] The recently reported structure of MmpL3 revealed resistance-conferring mutations to associate primarily with the transmembrane domain.[82] Although resistance to pre-clinical MmpL3 inhibitors has been detected, analysis of the widespread mutational landscape revealed a low level of environmental resistance.[82] This suggests that MmpL3 inhibitors currently undergoing clinical trials would face little resistance if made available. Additionally, the ability of many MmpL3 inhibitors to work synergistically with other antitubercular drugs presents a ray of hope in combatting the TB crisis.
Host genetics[edit]
The nature of the host-pathogen interaction between humans and M. tuberculosis is considered to have a genetic component. A group of rare disorders called Mendelian susceptibility to mycobacterial diseases was observed in a subset of individuals with a genetic defect that results in increased susceptibility to mycobacterial infection.[83]
Early case and twin studies have indicated that genetic components are important in host susceptibility to M. tuberculosis. Recent genome-wide association studies (GWAS) have identified three genetic risk loci, including at positions 11p13 and 18q11.[84][85] As is common in GWAS, the variants discovered have moderate effect sizes.
DNA repair[edit]
As an intracellular pathogen, M. tuberculosis is exposed to a variety of DNA-damaging assaults, primarily from host-generated antimicrobial toxic radicals. Exposure to reactive oxygen species and/or reactive nitrogen species causes different types of DNA damage including oxidation, depurination, methylation, and deamination that can give rise to single- and double-strand breaks (DSBs).
DnaE2 polymerase is upregulated in M. tuberculosis by several DNA-damaging agents, as well as during infection of mice.[86] Loss of this DNA polymerase reduces the virulence of M. tuberculosis in mice.[86] DnaE2 is an error-prone DNA repair polymerase that appears to contribute to M. tuberculosis survival during infection.
The two major pathways employed in repair of DSBs are homologous recombinational repair (HR) and nonhomologous end joining (NHEJ). Macrophage-internalized M. tuberculosis is able to persist if either of these pathways is defective, but is attenuated when both pathways are defective.[87] This indicates that intracellular exposure of M. tuberculosis to reactive oxygen and/or reactive nitrogen species results in the formation of DSBs that are repaired by HR or NHEJ.[87] However deficiency of DSB repair does not appear to impair M. tuberculosis virulence in animal models.[88]
History[edit]
M. tuberculosis, then known as the "tubercle bacillus", was first described on 24 March 1882 by Robert Koch, who subsequently received the Nobel Prize in Physiology or Medicine for this discovery in 1905; the bacterium is also known as "Koch's bacillus".[89][90]
M. tuberculosis has existed throughout history, but the name has changed frequently over time. In 1720, though, the history of tuberculosis started to take shape into what is known of it today; as the physician Benjamin Marten described in his A Theory of Consumption, tuberculosis may be caused by small living creatures transmitted through the air to other patients.[91]
Vaccine[edit]
The BCG vaccine (bacille Calmette-Guerin), which was derived from M. bovis, while effective against childhood and severe forms of tuberculosis, has limited success in preventing the most common form of the disease today, adult pulmonary tuberculosis.[92] Because of this, it is primarily used in high tuberculosis incidence regions, and is not a recommended vaccine in the United States due to the low risk of infection. To receive this vaccine in the United States, an individual is required to go through a consultation process with an expert in M. tuberculosis and is only given to those who meet the specific criteria.[93]
The BCG, according to an article of the Kyodo News (April 14, 2020) titled "Tuberculosis vaccine drawing attention in fight against coronavirus" indicates a possible correlation between BCG vaccination, and better immune response to the COVID-19.[94]
The DNA Vaccine, according to an article of the Journal of Preventive, Diagnostic and Treatment Strategies in Medicine ( September, 2022) "DNA Vaccine Construct Formation using Mycobacterium‑Specific Gene Inh‑A" specificies that DNA vaccine can be used alone or in combination with BCG. DNA vaccines have enough potential to be used with TB treatment and reduce the treatment time in future.[95]
See also[edit]
References[edit]
- ^ Gordon SV, Parish T (April 2018). "Microbe Profile: Mycobacterium tuberculosis: Humanity's deadly microbial foe". Microbiology. 164 (4): 437–439. doi:10.1099/mic.0.000601. PMID 29465344.
- ^ a b Ryan KJ, Ray CG (2004). "Mycobacteria". Sherris Medical Microbiology : an Introduction to Infectious Diseases (4th ed.). New York: McGraw-Hill. p. 439. ISBN 978-0-83-858529-0.
- ^ Fu LM, Fu-Liu CS (1 January 2002). "Is Mycobacterium tuberculosis a closer relative to Gram-positive or Gram-negative bacterial pathogens?". Tuberculosis. 82 (2–3): 85–90. doi:10.1054/tube.2002.0328. PMID 12356459.
- ^ a b Cudahy P, Shenoi SV (April 2016). "Diagnostics for pulmonary tuberculosis". Postgraduate Medical Journal. 92 (1086): 187–193. doi:10.1136/postgradmedj-2015-133278. PMC 4854647. PMID 27005271.
- ^ Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. (June 1998). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature. 393 (6685): 537–44. Bibcode:1998Natur.393..537C. doi:10.1038/31159. PMID 9634230.
- ^ Camus JC, Pryor MJ, Médigue C, Cole ST (October 2002). "Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv". Microbiology. 148 (Pt 10): 2967–73. doi:10.1099/00221287-148-10-2967. PMID 12368430.
- ^ a b c d e f g h i van Ingen J, Rahim Z, Mulder A, Boeree MJ, Simeone R, Brosch R, et al. (April 2012). "Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies". Emerging Infectious Diseases. 18 (4): 653–55. doi:10.3201/eid1804.110888. PMC 3309669. PMID 22469053.
- ^ Parish T, Stoker NG (December 1999). "Mycobacteria: bugs and bugbears (two steps forward and one step back)". Molecular Biotechnology. 13 (3): 191–200. doi:10.1385/MB:13:3:191. PMID 10934532. S2CID 28960959.
- ^ a b c Fitzgerald DW, Sterline TR, Haas DW (2015). "251 – Mycobacterium tuberculosis". In Bennett JE, Dolin R, Blaser MJ (eds.). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Elsevier Saunders. p. 2787. ISBN 978-1-4557-4801-3. OCLC 903327877.
- ^ Murray PR, Rosenthal KS, Pfaller MA (2005). Medical Microbiology. Elsevier Mosby.
- ^ a b Hunter RL, Olsen MR, Jagannath C, Actor JK (2006). "Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease". Annals of Clinical and Laboratory Science. 36 (4): 371–386. PMID 17127724.
- ^ Todar K. "Mycobacterium tuberculosis and Tuberculosis". textbookofbacteriology.net. Retrieved 24 December 2016.
- ^ McMurray DN (1996). "Mycobacteria and Nocardia". In Baron S (ed.). Medical Microbiology (4th ed.). Galveston (TX): University of Texas Medical Branch at Galveston. ISBN 978-0963117212. PMID 21413269.
- ^ Bicmen C, Gunduz AT, Coskun M, Senol G, Cirak AK, Ozsoz A (August 2011). "Molecular detection and identification of mycobacterium tuberculosis complex and four clinically important nontuberculous mycobacterial species in smear-negative clinical samples by the genotype mycobacteria direct test". Journal of Clinical Microbiology. 49 (8): 2874–78. doi:10.1128/JCM.00612-11. PMC 3147717. PMID 21653780.
- ^ Saleeb PG, Drake SK, Murray PR, Zelazny AM (May 2011). "Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry". Journal of Clinical Microbiology. 49 (5): 1790–94. doi:10.1128/JCM.02135-10. PMC 3122647. PMID 21411597.
- ^ a b c Yamada H, Yamaguchi M, Chikamatsu K, Aono A, Mitarai S (28 January 2015). "Structome analysis of virulent Mycobacterium tuberculosis, which survives with only 700 ribosomes per 0.1 fl of cytoplasm". PLOS ONE. 10 (1): e0117109. Bibcode:2015PLoSO..1017109Y. doi:10.1371/journal.pone.0117109. PMC 4309607. PMID 25629354.
- ^ "How TB Spreads". Center for Disease Control. 26 July 2016. Retrieved 14 March 2018.
- ^ Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, et al. (January 1997). "Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis". Infection and Immunity. 65 (1): 298–304. doi:10.1128/IAI.65.1.298-304.1997. PMC 174591. PMID 8975927.
- ^ Mann FM, Xu M, Chen X, Fulton DB, Russell DG, Peters RJ (December 2009). "Edaxadiene: a new bioactive diterpene from Mycobacterium tuberculosis". Journal of the American Chemical Society. 131 (48): 17526–27. doi:10.1021/ja9019287. PMC 2787244. PMID 19583202.
- ^ Flynn JL, Chan J (August 2003). "Immune evasion by Mycobacterium tuberculosis: living with the enemy". Current Opinion in Immunology. 15 (4): 450–55. doi:10.1016/S0952-7915(03)00075-X. PMID 12900278.
- ^ Buter J, Cheng TY, Ghanem M, Grootemaat AE, Raman S, Feng X, et al. (September 2019). "Mycobacterium tuberculosis releases an antacid that remodels phagosomes". Nature Chemical Biology. 15 (9): 889–899. doi:10.1038/s41589-019-0336-0. PMC 6896213. PMID 31427817.
- ^ Brodin P, Hoffmann E (September 2019). "T(oo)bAd". Nature Chemical Biology. 15 (9): 849–850. doi:10.1038/s41589-019-0347-x. PMID 31427816. S2CID 209569609.
- ^ a b c d Schaaf K, Smith SR, Duverger A, Wagner F, Wolschendorf F, Westfall AO, et al. (February 2017). "Mycobacterium tuberculosis exploits the PPM1A signaling pathway to block host macrophage apoptosis". Scientific Reports. 7: 42101. Bibcode:2017NatSR...742101S. doi:10.1038/srep42101. PMC 5296758. PMID 28176854.
- ^ Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH (November 2013). "Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing". Clinical and Experimental Immunology. 174 (2): 193–202. doi:10.1111/cei.12170. PMC 3828822. PMID 23841514.
- ^ Ramakrishnan L (April 2012). "Revisiting the role of the granuloma in tuberculosis". Nature Reviews. Immunology. 12 (5): 352–366. doi:10.1038/nri3211. PMID 22517424. S2CID 1139969.
- ^ Marakalala MJ, Raju RM, Sharma K, Zhang YJ, Eugenin EA, Prideaux B, et al. (May 2016). "Inflammatory signaling in human tuberculosis granulomas is spatially organized". Nature Medicine. 22 (5): 531–538. doi:10.1038/nm.4073. PMC 4860068. PMID 27043495.
- ^ Gern BH, Adams KN, Plumlee CR, Stoltzfus CR, Shehata L, Moguche AO, et al. (April 2021). "TGFβ restricts expansion, survival, and function of T cells within the tuberculous granuloma". Cell Host & Microbe. 29 (4): 594–606.e6. doi:10.1016/j.chom.2021.02.005. PMC 8624870. PMID 33711270. S2CID 232217715.
- ^ Davis JM, Ramakrishnan L (January 2009). "The role of the granuloma in expansion and dissemination of early tuberculous infection". Cell. 136 (1): 37–49. doi:10.1016/j.cell.2008.11.014. PMC 3134310. PMID 19135887.
- ^ Cohen SB, Gern BH, Urdahl KB (April 2022). "The Tuberculous Granuloma and Preexisting Immunity". Annual Review of Immunology. 40 (1): 589–614. doi:10.1146/annurev-immunol-093019-125148. PMID 35130029. S2CID 246651980.
- ^ Wooldridge K, ed. (2009). Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press. ISBN 978-1-904455-42-4.
- ^ Schaaf K, Hayley V, Speer A, Wolschendorf F, Niederweis M, Kutsch O, et al. (August 2016). "A Macrophage Infection Model to Predict Drug Efficacy Against Mycobacterium Tuberculosis". Assay and Drug Development Technologies. 14 (6): 345–354. doi:10.1089/adt.2016.717. PMC 4991579. PMID 27327048.
- ^ Takekawa M, Maeda T, Saito H (August 1998). "Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways". The EMBO Journal. 17 (16): 4744–52. doi:10.1093/emboj/17.16.4744. PMC 1170803. PMID 9707433.
- ^ Dhanasekaran DN, Reddy EP (October 2008). "JNK signaling in apoptosis". Oncogene. 27 (48): 6245–51. doi:10.1038/onc.2008.301. PMC 3063296. PMID 18931691.
- ^ The ability to restore macrophage apoptosis to M. tuberculosis infected ones could improve the current tuberculosis chemotherapy treatment, as TB drugs can gain better access to the bacteria in the niche (M),
- ^ "Tuberculosis – Symptoms and causes". Mayo Clinic. Retrieved 12 November 2019.
- ^ Zhang Y, Mazurek GH, Cave MD, Eisenach KD, Pang Y, Murphy DT, et al. (June 1992). "DNA polymorphisms in strains of Mycobacterium tuberculosis analyzed by pulsed-field gel electrophoresis: a tool for epidemiology". Journal of Clinical Microbiology. 30 (6): 1551–56. doi:10.1128/JCM.30.6.1551-1556.1992. PMC 265327. PMID 1352518.
- ^ Frothingham R, Meeker-O'Connell WA (May 1998). "Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats". Microbiology. 144 (Pt 5): 1189–96. doi:10.1099/00221287-144-5-1189. PMID 9611793.
- ^ Mazars E, Lesjean S, Banuls AL, Gilbert M, Vincent V, Gicquel B, et al. (February 2001). "High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology". Proceedings of the National Academy of Sciences of the United States of America. 98 (4): 1901–06. Bibcode:2001PNAS...98.1901M. doi:10.1073/pnas.98.4.1901. PMC 29354. PMID 11172048.
- ^ Hawkey PM, Smith EG, Evans JT, Monk P, Bryan G, Mohamed HH, et al. (August 2003). "Mycobacterial interspersed repetitive unit typing of Mycobacterium tuberculosis compared to IS6110-based restriction fragment length polymorphism analysis for investigation of apparently clustered cases of tuberculosis". Journal of Clinical Microbiology. 41 (8): 3514–20. doi:10.1128/JCM.41.8.3514-3520.2003. PMC 179797. PMID 12904348.
- ^ Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, et al. (December 2006). "Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis". Journal of Clinical Microbiology. 44 (12): 4498–510. doi:10.1128/JCM.01392-06. PMC 1698431. PMID 17005759.
- ^ Müller R, Roberts CA, Brown TA (2015). "Complications in the study of ancient tuberculosis: non-specificity of IS6110 PCRs". Science and Technology of Archaeological Research. 1 (1): 1–8. Bibcode:2015STAR....1....1M. doi:10.1179/2054892314Y.0000000002.
- ^ Rattan A, Kalia A, Ahmad N (June 1998). "Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives". Emerging Infectious Diseases. 4 (2): 195–209. doi:10.3201/eid0402.980207. PMC 2640153. PMID 9621190.
- ^ "Drug-resistant TB". Center for Disease Control. April 2014.
- ^ Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. (June 1998). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature. 393 (6685): 537–44. Bibcode:1998Natur.393..537C. doi:10.1038/31159. PMID 9634230.
- ^ "Mycobacterium tuberculosis". Sanger Institute. 29 March 2007. Retrieved 16 November 2008.
- ^ Bloch H, Segal W (August 1956). "Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro". Journal of Bacteriology. 72 (2): 132–41. doi:10.1128/JB.72.2.132-141.1956. PMC 357869. PMID 13366889.
- ^ Wipperman MF, Sampson NS, Thomas ST (2014). "Pathogen roid rage: cholesterol utilization by Mycobacterium tuberculosis". Critical Reviews in Biochemistry and Molecular Biology. 49 (4): 269–93. doi:10.3109/10409238.2014.895700. PMC 4255906. PMID 24611808.
- ^ Glickman MS, Jacobs WR (February 2001). "Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline". Cell. 104 (4): 477–85. doi:10.1016/S0092-8674(01)00236-7. PMID 11239406. S2CID 11557497.
- ^ Arnvig KB, Young DB (August 2009). "Identification of small RNAs in Mycobacterium tuberculosis". Molecular Microbiology. 73 (3): 397–408. doi:10.1111/j.1365-2958.2009.06777.x. PMC 2764107. PMID 19555452.
- ^ Livny J, Brencic A, Lory S, Waldor MK (2006). "Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2". Nucleic Acids Research. 34 (12): 3484–93. doi:10.1093/nar/gkl453. PMC 1524904. PMID 16870723.
- ^ Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, et al. (October 2013). "Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance". Nature Genetics. 45 (10): 1255–60. doi:10.1038/ng.2735. PMID 23995137. S2CID 14396673.
- ^ a b Shell SS, Prestwich EG, Baek SH, Shah RR, Sassetti CM, Dedon PC, et al. (4 July 2013). "DNA methylation impacts gene expression and ensures hypoxic survival of Mycobacterium tuberculosis". PLOS Pathogens. 9 (7): e1003419. doi:10.1371/journal.ppat.1003419. PMC 3701705. PMID 23853579.
- ^ Zhu L, Zhong J, Jia X, Liu G, Kang Y, Dong M, et al. (January 2016). "Precision methylome characterization of Mycobacterium tuberculosis complex (MTBC) using PacBio single-molecule real-time (SMRT) technology". Nucleic Acids Research. 44 (2): 730–743. doi:10.1093/nar/gkv1498. PMC 4737169. PMID 26704977.
- ^ a b c d e f Modlin SJ, Conkle-Gutierrez D, Kim C, Mitchell SN, Morrissey C, Weinrick BC, et al. (October 2020). Stallings CL, Soldati-Favre D, Casadesús J (eds.). "Drivers and sites of diversity in the DNA adenine methylomes of 93 Mycobacterium tuberculosis complex clinical isolates". eLife. 9: e58542. doi:10.7554/eLife.58542. PMID 33107429.
- ^ Phelan J, de Sessions PF, Tientcheu L, Perdigao J, Machado D, Hasan R, et al. (January 2018). "Methylation in Mycobacterium tuberculosis is lineage specific with associated mutations present globally". Scientific Reports. 8 (1): 160. Bibcode:2018NatSR...8..160P. doi:10.1038/s41598-017-18188-y. PMID 29317751.
- ^ Blow MJ, Clark TA, Daum CG, Deutschbauer AM, Fomenkov A, Fries R, et al. (February 2016). "The Epigenomic Landscape of Prokaryotes". PLOS Genetics. 12 (2): e1005854. doi:10.1371/journal.pgen.1005854. PMC 4752239. PMID 26870957.
- ^ Beaulaurier J, Zhang XS, Zhu S, Sebra R, Rosenbluh C, Deikus G, et al. (June 2015). "Single molecule-level detection and long read-based phasing of epigenetic variations in bacterial methylomes". Nature Communications. 6 (1): 7438. Bibcode:2015NatCo...6.7438B. doi:10.1038/ncomms8438. PMC 4490391. PMID 26074426.
- ^ a b Blouin Y, Hauck Y, Soler C, Fabre M, Vong R, Dehan C, et al. (2012). "Significance of the identification in the Horn of Africa of an exceptionally deep branching Mycobacterium tuberculosis clade". PLOS ONE. 7 (12): e52841. Bibcode:2012PLoSO...752841B. doi:10.1371/journal.pone.0052841. PMC 3531362. PMID 23300794.
- ^ a b Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. (October 2013). "Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans". Nature Genetics. 45 (10): 1176–82. doi:10.1038/ng.2744. PMC 3800747. PMID 23995134.
- ^ Blouin Y, Cazajous G, Dehan C, Soler C, Vong R, Hassan MO, et al. (January 2014). "Progenitor "Mycobacterium canettii" clone responsible for lymph node tuberculosis epidemic, Djibouti". Emerging Infectious Diseases. 20 (1): 21–28. doi:10.3201/eid2001.130652. PMC 3884719. PMID 24520560.
- ^ a b Galagan JE (May 2014). "Genomic insights into tuberculosis". Nature Reviews. Genetics. 15 (5): 307–20. doi:10.1038/nrg3664. PMID 24662221. S2CID 7371757.
- ^ Malm S, Linguissi LS, Tekwu EM, Vouvoungui JC, Kohl TA, Beckert P, et al. (March 2017). "New Mycobacterium tuberculosis Complex Sublineage, Brazzaville, Congo". Emerging Infectious Diseases. 23 (3): 423–29. doi:10.3201/eid2303.160679. PMC 5382753. PMID 28221129.
- ^ Wirth T, Hildebrand F, Allix-Béguec C, Wölbeling F, Kubica T, Kremer K, et al. (September 2008). "Origin, spread and demography of the Mycobacterium tuberculosis complex". PLOS Pathogens. 4 (9): e1000160. doi:10.1371/journal.ppat.1000160. PMC 2528947. PMID 18802459.
- ^ Eldholm V, Pettersson JH, Brynildsrud OB, Kitchen A, Rasmussen EM, Lillebaek T, et al. (November 2016). "Armed conflict and population displacement as drivers of the evolution and dispersal of Mycobacterium tuberculosis". Proceedings of the National Academy of Sciences of the United States of America. 113 (48): 13881–86. Bibcode:2016PNAS..11313881E. doi:10.1073/pnas.1611283113. PMC 5137683. PMID 27872285.
- ^ Bos KI, Harkins KM, Herbig A, Coscolla M, Weber N, Comas I, et al. (October 2014). "Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis". Nature. 514 (7523): 494–497. Bibcode:2014Natur.514..494B. doi:10.1038/nature13591. PMC 4550673. PMID 25141181.
- ^ Loiseau C, Menardo F, Aseffa A, Hailu E, Gumi B, Ameni G, Berg S, Rigouts L, Robbe-Austerman S, Zinsstag J, Gagneux S, Brites D (2020) An African origin for Mycobacterium bovis. Evol Med Public Health. 2020 Jan 31;2020(1):49–59
- ^ Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, et al. (February 2006). "Variable host-pathogen compatibility in Mycobacterium tuberculosis". Proceedings of the National Academy of Sciences of the United States of America. 103 (8): 2869–73. Bibcode:2006PNAS..103.2869G. doi:10.1073/pnas.0511240103. PMC 1413851. PMID 16477032.
- ^ Hirsh AE, Tsolaki AG, DeRiemer K, Feldman MW, Small PM (April 2004). "Stable association between strains of Mycobacterium tuberculosis and their human host populations". Proceedings of the National Academy of Sciences of the United States of America. 101 (14): 4871–76. doi:10.1073/pnas.0305627101. PMC 387341. PMID 15041743.
- ^ Pepperell CS, Casto AM, Kitchen A, Granka JM, Cornejo OE, Holmes EC, et al. (August 2013). "The role of selection in shaping diversity of natural M. tuberculosis populations". PLOS Pathogens. 9 (8): e1003543. doi:10.1371/journal.ppat.1003543. PMC 3744410. PMID 23966858.
- ^ a b c Bos KI, Harkins KM, Herbig A, Coscolla M, Weber N, Comas I, et al. (October 2014). "Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis". Nature. 514 (7523): 494–97. Bibcode:2014Natur.514..494B. doi:10.1038/nature13591. PMC 4550673. PMID 25141181.
- ^ Kay GL, Sergeant MJ, Zhou Z, Chan JZ, Millard A, Quick J, et al. (April 2015). "Eighteenth-century genomes show that mixed infections were common at time of peak tuberculosis in Europe". Nature Communications. 6 (1): 6717. Bibcode:2015NatCo...6.6717K. doi:10.1038/ncomms7717. PMC 4396363. PMID 25848958.
- ^ a b Brynildsrud OB, Pepperell CS, Suffys P, Grandjean L, Monteserin J, Debech N, et al. (October 2018). "Mycobacterium tuberculosis lineage 4 shaped by colonial migration and local adaptation". Science Advances. 4 (10): eaat5869. doi:10.1126/sciadv.aat5869. PMC 6192687. PMID 30345355.
- ^ Gutierrez MC, Brisse S, Brosch R, Fabre M, Omaïs B, Marmiesse M, et al. (September 2005). "Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis". PLOS Pathogens. 1 (1): e5. doi:10.1371/journal.ppat.0010005. PMC 1238740. PMID 16201017.
- ^ Hershkovitz I, Donoghue HD, Minnikin DE, Besra GS, Lee OY, Gernaey AM, et al. (15 October 2008). Ahmed N (ed.). "Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean". PLOS ONE. 3 (10). Public Library of Science (PLoS): e3426. Bibcode:2008PLoSO...3.3426H. doi:10.1371/journal.pone.0003426. PMC 2565837. PMID 18923677.
- ^ Eldholm V, Balloux F (August 2016). "Antimicrobial Resistance in Mycobacterium tuberculosis: The Odd One Out". Trends in Microbiology. 24 (8): 637–648. doi:10.1016/j.tim.2016.03.007. PMID 27068531.
- ^ a b c d e Global tuberculosis report 2020. World Health Organization. 2020. ISBN 978-92-4-001313-1. OCLC 1258341826.
- ^ Ou ZJ, Yu DF, Liang YH, He WQ, Li YZ, Meng YX, et al. (March 2021). "Trends in burden of multidrug-resistant tuberculosis in countries, regions, and worldwide from 1990 to 2017: results from the Global Burden of Disease study". Infectious Diseases of Poverty. 10 (1): 24. doi:10.1186/s40249-021-00803-w. PMC 7936417. PMID 33676581.
- ^ Zaw MT, Emran NA, Lin Z (September 2018). "Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis". Journal of Infection and Public Health. 11 (5): 605–610. doi:10.1016/j.jiph.2018.04.005. PMID 29706316. S2CID 14058414.
- ^ a b Palomino JC, Martin A (July 2014). "Drug Resistance Mechanisms in Mycobacterium tuberculosis". Antibiotics. 3 (3): 317–340. doi:10.3390/antibiotics3030317. PMC 4790366. PMID 27025748.
- ^ Chalut C (September 2016). "MmpL transporter-mediated export of cell-wall associated lipids and siderophores in mycobacteria". Tuberculosis. 100: 32–45. doi:10.1016/j.tube.2016.06.004. PMID 27553408.
- ^ a b c Xu Z, Meshcheryakov VA, Poce G, Chng SS (July 2017). "MmpL3 is the flippase for mycolic acids in mycobacteria". Proceedings of the National Academy of Sciences of the United States of America. 114 (30): 7993–7998. Bibcode:2017PNAS..114.7993X. bioRxiv 10.1101/099440. doi:10.1073/pnas.1700062114. PMC 5544280. PMID 28698380.
- ^ a b Adams O, Deme JC, Parker JL, Fowler PW, Lea SM, Newstead S (October 2021). "Cryo-EM structure and resistance landscape of M. tuberculosis MmpL3: An emergent therapeutic target". Structure. 29 (10): 1182–1191.e4. doi:10.1016/j.str.2021.06.013. PMC 8752444. PMID 34242558.
- ^ Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL (December 2014). "Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity". Seminars in Immunology. 26 (6): 454–70. doi:10.1016/j.smim.2014.09.008. PMC 4357480. PMID 25453225.
- ^ Thye T, Owusu-Dabo E, Vannberg FO, van Crevel R, Curtis J, Sahiratmadja E, et al. (February 2012). "Common variants at 11p13 are associated with susceptibility to tuberculosis". Nature Genetics. 44 (3): 257–59. doi:10.1038/ng.1080. PMC 3427019. PMID 22306650.
- ^ Thye T, Vannberg FO, Wong SH, Owusu-Dabo E, Osei I, Gyapong J, et al. (September 2010). "Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2". Nature Genetics. 42 (9): 739–41. doi:10.1038/ng.639. PMC 4975513. PMID 20694014.
- ^ a b Boshoff HI, Reed MB, Barry CE, Mizrahi V (April 2003). "DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis". Cell. 113 (2): 183–93. doi:10.1016/s0092-8674(03)00270-8. PMID 12705867. S2CID 6273732.
- ^ a b Brzostek A, Szulc I, Klink M, Brzezinska M, Sulowska Z, Dziadek J (2014). "Either non-homologous ends joining or homologous recombination is required to repair double-strand breaks in the genome of macrophage-internalized Mycobacterium tuberculosis". PLOS ONE. 9 (3): e92799. Bibcode:2014PLoSO...992799B. doi:10.1371/journal.pone.0092799. PMC 3962454. PMID 24658131.
- ^ Heaton BE, Barkan D, Bongiorno P, Karakousis PC, Glickman MS (August 2014). "Deficiency of double-strand DNA break repair does not impair Mycobacterium tuberculosis virulence in multiple animal models of infection". Infection and Immunity. 82 (8): 3177–85. doi:10.1128/IAI.01540-14. PMC 4136208. PMID 24842925.
- ^ "Robert Koch and Tuberculosis: Koch's Famous Lecture". Nobel Foundation. 2008. Retrieved 18 November 2008.
- ^ Scientific American. Munn & Company. 13 May 1882. p. 289.
- ^ "Tuberculosis History Timeline". Archived from the original on 21 June 2010. Retrieved 18 June 2010.
- ^ Herzmann C, Sotgiu G, Schaberg T, Ernst M, Stenger S, Lange C (October 2014). "Early BCG vaccination is unrelated to pulmonary immunity against Mycobacterium tuberculosis in adults". The European Respiratory Journal. 44 (4): 1087–1090. doi:10.1183/09031936.00086514. PMID 24969658. S2CID 12150010.
- ^ "Fact Sheets | Infection Control & Prevention | Fact Sheet – BCG Vaccine | TB | CDC". www.cdc.gov. 11 December 2018. Retrieved 12 November 2019.
- ^ "Tuberculosis vaccine drawing attention in fight against coronavirus". Kyodo News+.
- ^ Anwar S, Qureshi J, Shahzad MI, Zaman M, Jilani A (2022). "DNA vaccine construct formation using Mycobacterium-specific gene Inh-A". Journal of Preventive, Diagnostic and Treatment Strategies in Medicine. 1 (3): 192. doi:10.4103/jpdtsm.jpdtsm_63_22. ISSN 2949-6594.
