Wikipedia:Reference desk/Archives/Science/2008 February 5
| Science desk | ||
|---|---|---|
| < February 4 | << Jan | February | Mar >> | February 6 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 5[edit]
Need phase diagram for CO2 below 1 atm, please?[edit]
I am trying to find out the freezing point of CO2 at .62 atm, and all the diagrams I find are skewed to high pressures with 1 atm way down in the corner!
Does anybody have a good diagram for low pressures? --BenBurch (talk) 03:41, 5 February 2008 (UTC)
The best one I could find came from a link on wikipedia, go figure. Because your looking at C02 at a pressure of .62 atm, the boiling point is very low(same with water). Because of this most sites probally find it pointless to go below 1 atm because it is a gas most of the time. (you have to get below about 100 degrees C. Anyway this is the best graph I could find. good luck,--Pewwer42 Talk 05:04, 5 February 2008 (UTC)
- It's remarkably hard to find figures or tables giving the data you want. However, I did finally find the temperature range you were interested in in a 1937 Journal of Chemical Physics article (W. F. Giauque and C. J. Egan). It gives a model for the sublimation pressure (as a function of temperature) for the range 154-196 kelvin. The article is here, but you may need a subscription - not sure. The model given was (sorry, too lazy to format it): log10 P(int. cm Hg) = — (1354.210/T)+8.69903+0.0015880T—4.5107×10—6T2, which by my calculations (after changing the units around) results in a sublimation temperature of 189.19 kelvin at 0.63 atmospheres. I made a little figure
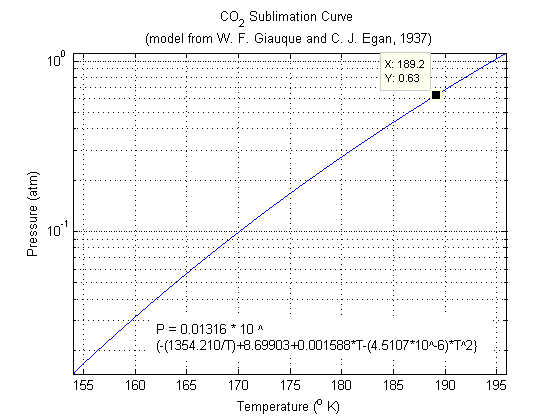 . --Bmk (talk) 06:06, 5 February 2008 (UTC)
. --Bmk (talk) 06:06, 5 February 2008 (UTC)
Thanks so much! --BenBurch (talk) —Preceding comment was added at 06:32, 5 February 2008 (UTC)
- PS - I accidentally misread your question and answered for 0.63 atmospheres. The value you were looking for is actually 189.01 Kelvin at 0.62 atmospheres. Sorry! --Bmk (talk) 20:43, 5 February 2008 (UTC)
Components of remote controlled battery powered car[edit]
Through the great service of Wikipedia ,i want to know about remote controlled battery powered car,because i am new in this field but have a strong desire to do something.So,please help me. thank you —Preceding unsigned comment added by Ankurrai2008 (talk • contribs) 07:41, 5 February 2008 (UTC)
- Welcome to Wikipedia. You can easily look up this topic yourself. Please see Radio-controlled car. For future questions, try using the search box at the top left of the screen. It's much quicker, and you will probably find a clearer answer. If you still don't understand, add a further question below by clicking the "edit" button to the right of your question title. --Shantavira|feed me 08:31, 5 February 2008 (UTC)
semen increasing foods[edit]
Is there any foods or natural supplements who REALLY work to increase semen quantity 203.128.27.61 (talk) 08:26, 5 February 2008 (UTC)
- I think if there was it would be very, very, well known. A bit like remedies for haemorrhoids, the common cold and increasing your manhood - lots of adverts, claims and "I had this friend" stories but few reliable results. I will bet that someone will be by here in a while with a 'surefire' solution to your question. Richard Avery (talk) 14:05, 5 February 2008 (UTC)
- This is about the 5th time I've read the question. And I don't even check out the RDS all the time... Sorry to hijack this question but I still have absolutely no idea why people seem to be interested in this. Short of there being lots of aspiring Peter North (actor) pornstars I fail to see the attraction. Other then perhaps a longer orgasm it seems somewhat pointless to me. I've seen no evidence whatsoever that it has any effect on parter preference or pleasure (at least with penis size there is an understandable reason why people may feel it is important even if the evidence suggests it's not nearly as important as people think it is) and indeed it's something that very often may not be noticed (condoms, internal ejaculation etc) by the other partner (and when it does, it's I expect far more likely to be more annoying then welcomed/prefered). If it's to do with fertility, as I say everytime I see this, you should see a doctor if you have fertility concerns and more importantly, upping your semen level on it's on is pretty pointless. There's a very good chance all you're going to do is to dilute your sperm i.e. you end up with the same total sperm count in the end. What you need to do is to increase your total sperm count (and ensure you have healthy sperm), whether or not that increases your semen quantity is (mostly) immaterial. N.B. I say mostly because semen obviously plays a role in male fertility. However I've never seen any evidence it's much of an issue most of the time, all I've seen suggests that total sperm count and sperm health are what matters the most and are mostly likely to be a problem. An exceptionally low semen level would probably be a problem but I expect that beyond that the effects are minimal beyond the fact that a person with a lowish semen level MAY have on average a lower total sperm count then a person with a highish semen level (but do remember that just because there's a correlation doesn't mean your going to do anything by increasing the semen level since the fact remains that's not the primary problem). Nil Einne (talk) 11:50, 6 February 2008 (UTC)
Dowson gas[edit]
We are developing an anthracite mine in South Africa, and I am investigating the possibility of generating own power through the production of Dowson gas, using the anthracite both for steam generation and as carbon source. Please provide me with info with respect to: - Efficiency of the process - Available plants - Anyone else currently doing it —Preceding unsigned comment added by 155.239.195.40 (talk) 08:40, 5 February 2008 (UTC)
- coal gas would be the starting point and goggle scholar should provide some lierature.--Stone (talk) 11:24, 5 February 2008 (UTC)
True or False: Gravity Travels at the Speed of Light[edit]
Does gravity travel at the speed of light? —Preceding unsigned comment added by 212.51.122.6 (talk) 10:03, 5 February 2008 (UTC)
- Yes. See speed of gravity article and Science Reference Desk discussion on February 1. Gandalf61 (talk) 10:40, 5 February 2008 (UTC)
- This is actually a very complicated and subtle question. The gravitational field of a massive object doesn't travel at the speed of light or at any other speed; it's just there. If anything you could say that it travels at the same speed as the object (probably slower than light). A lot of people get confused into thinking that the gravitational field of a moving object lags behind the object's motion. In fact it follows directly from the principle of relativity that it can't lag behind, because there's no meaningful "behind" for it to lag in. Harder to explain but also true is that a uniformly accelerating object doesn't have any field lag. The reason for this is another principle like the principle of relativity; I don't know if it has a name, but it's related to the same spacetime symmetry that's the basis for Rindler coordinates. But there's no corresponding symmetry for jerk, which is a change in acceleration. That does cause field lag, also known as gravitational radiation or gravitational waves. Gravitational radiation propagates outward at the speed of light—but it's not even immediately clear what that means, given that gravitational radiation is a distortion of the spacetime metric, and it's only with respect to the spacetime metric that you can talk about speed in the first place. I think it was controversial for a long time whether general relativity predicted gravitational waves at all, or if it did whether they would travel at c or at some other speed, but that issue is now settled experimentally and I think settled theoretically as well. My blithe statement above that uniformly accelerated charges don't radiate glossed over some treacherous territory. Stephen Parrott believes that they do radiate, which would be an experimentally testable violation of the equivalence principle. He's gotta be wrong, but it's hard to say exactly why. Even in the much simpler theory of classical electromagnetism there are very tricky questions surrounding radiation from point charges which have yet to be resolved. Similar problems in quantum field theory were solved (or at least worked around) by renormalization, but gravity isn't renormalizable. So, this is a very hard problem and nobody really understands what's going on. -- BenRG (talk) 13:30, 5 February 2008 (UTC)
Prisoner's vision[edit]
Is there such a thing in psychology and neurology as 'Prisoner's vision'? How does, being enclosed in a small space with no line of horizon affect your vision and what are the results when going outside for the first time? How do these changes affect ones psychological state? Thank you. Keria (talk) 11:04, 5 February 2008 (UTC)
Maybe this, no? Sensory deprivation Fribbler (talk) 16:55, 5 February 2008 (UTC)
- Related (since it is based on limited concept of distance)... I read a book on pygmies in Africa that, according to the book, had no concept of great distance because they lived in the forest and never saw the horizon. It claimed that they didn't believe it when they were told the animal on the other side of a large plain was an elephant because it was far too small. I later read an article claiming that the book was based on falsified studies. So, if you do a search for studies on African pygmies and limited concept of distance, you will probably turn of controversial information. -- kainaw™ 17:41, 5 February 2008 (UTC)
how can we tell "shiny black" from "grey"[edit]
if a color of an object is based on what it absorbs, and color in total is a sum of the different wavelengths of light, then why can we tell that something is black, but shiny. shouldn't the "shiny" part just make it look grey instead of black? After all, if you take something black-looking, and mix it with a little white (the shiny part) you get grey...
so why don't we get grey instead of shiny black? —Preceding unsigned comment added by 212.51.122.6 (talk) 11:12, 5 February 2008 (UTC)
- Shinyness isn't so much about the ammount of reflection (and pure black basically doesn't exist) as the type of reflection. A matte object scatters light roughly equally in all directions. A shiney object has a smooth surface which reflects more like a mirror with most of the incoming light from a particular source leaving in the same direction. It is the highlights this produces that make us describe an object as shiny. Plugwash (talk) 11:27, 5 February 2008 (UTC)
- Why doesn't pure black exist??? What about a room completely free of light, isn't that 'pure black'?
- I believe Plugwash is referring to black as a colour of objects. Thus he means that there are basically no objects that absorb all light and reflect none. Algebraist 13:19, 5 February 2008 (UTC)
- Why doesn't pure black exist??? What about a room completely free of light, isn't that 'pure black'?
- There is some information in the article diffuse reflection, and in some links therein. Diffuse reflection of light from objects (the technical term for why you can see the color of objects) is as complicated as the surface of the object; the quality of the reflection has a lot to do with the nature of the surface texture. In terms of "pure black", I think that Plugwash was pointing out that in practice, no surface absorbs all incident light without reradiating any of it, and in practice it is impossible to have a room with no light (as a point of interest, it is also impossible in theory! See Vacuum energy.) --Bmk (talk) 13:23, 5 February 2008 (UTC)
Along with diffuse reflection (already mentioned), you might also want to see our article about specular reflection. And then there are these two external links: [1] and [2].
Atlant (talk) 15:00, 5 February 2008 (UTC)
- But, if we could have pure black, then it couldn't reflect, right? Zrs 12 (talk) 15:03, 5 February 2008 (UTC)
- Yes, that's the definition of an ideal black body. However, no such bodies exist in nature (except maybe black holes, and I might be wrong about them). Algebraist 15:11, 5 February 2008 (UTC)
- Black holes emmitt Hawking radiation. Zrs 12 (talk) 15:15, 5 February 2008 (UTC)
- And while ideal black bodies do not reflect, they emit blackbody radiation. --Stephan Schulz (talk) 15:23, 5 February 2008 (UTC)
- On the subject of pure black - have you never seen laser printer toner?87.102.114.230 (talk) 18:57, 5 February 2008 (UTC)
- And while ideal black bodies do not reflect, they emit blackbody radiation. --Stephan Schulz (talk) 15:23, 5 February 2008 (UTC)
- Black holes emmitt Hawking radiation. Zrs 12 (talk) 15:15, 5 February 2008 (UTC)
- Yes, that's the definition of an ideal black body. However, no such bodies exist in nature (except maybe black holes, and I might be wrong about them). Algebraist 15:11, 5 February 2008 (UTC)
- But, if we could have pure black, then it couldn't reflect, right? Zrs 12 (talk) 15:03, 5 February 2008 (UTC)
I'm sorry that I don't have a reference, but I recall seeing a research paper a short time back which looked at this issue. Specifically, how do we tell the difference between a computer picture of a shiny object and of a gray object. With the computer picture, there is no polarization or "shininess" information stored - just brightness level (for B&W picture). IIRC, although the total amount of light (the total brightness level) may be the same, the distribution of brightness is different. That is, if you look at a histogram of brightness level vs. number of pixels, the curve for a shiny ball is skewed with respect to that of a matte gray ball. Supposedly, our brains are picking up on this skewed brightness distribution and flagging that as "shiny". -- 128.104.112.19 (talk) 19:34, 5 February 2008 (UTC)

Whether we perceive something as white, gray, or black depends mostly on how its brightness compares to things immediately adjacent to it. The Moon absorbs enough light (about 90%) that its color can reasonably be called black: look at the moon rock on display at the National Air and Space Museum, for example. But when you see the Moon adjacent to the night sky, which gives off no light at all, you see it as white (or part white and part light gray). Another simple demonstration is the same color illusion, shown at right: the dark gray square A and the light gray square B are actually giving off exactly the same amount of light, but you perceive B as lighter because its surroundings are darker. Further, the illustration also shows why you perceive things that way: it keeps you from being confused any time you have to look at something that's in shadow. --Anonymous, 00:33 UTC, February 6, 2008.
Calculating the phases of the Moon[edit]
How can I calculate the Moon phase for any given date? And/or calculate the dates for the extremes, that is full and new Moon. I’ve found many online services, but I’m interested in doing the calculations myself (and understanding all the steps while doing so). Kess (talk) 12:16, 5 February 2008 (UTC)
- Find a reference date which has a full moon. This will repeat every 29.53 days. 14.77 days after every full moon there will be a new moon. 7.38 days after a full moon the moon will be in the last quarter, and 7.38 days after a new moon, it will be in the first quarter. For short-term calculations, you can assume that the phases repeat roughly every 4 weeks, and that every change of phases from new to first, full, and last will take a week. --Stephan Schulz (talk) 12:25, 5 February 2008 (UTC)
- Thanks, but not really not what I was after; should have been more specific. With the answer above I would further like to calculate the date of the full moon myself, rather than using a reference. So, perhaps, the more specific question should be: How do I calculate the time of any specific full/new moon?
- (I have been nosing around the Kepler's laws of planetary motion article, thinking that if I could calculate the position of the Sun and the Moon relative Earth I could easily find the time for any Moon phase. But my astronomical-mathematical knowledge is too fragile, and I cannot figure out the needed calculations.)
- Kess (talk) 12:47, 5 February 2008 (UTC)
- You're going to need at least one reference datum to get you started. If you want, you can go out and observe a full moon, rather than looking one up. Algebraist 13:17, 5 February 2008 (UTC)
- You need more than that. Neither the Moon's orbit nor the Earth's is circular, which means that the number 29.53 mentioned above is only an average, not a constant. Fred Espenak's eclipse pages also include a 6,000-year table of lunar phases, in which you can see here that the next six full moons will be at these UTC times. (For other centuries, follow the "catalog" link at the bottom.) At right I have added the time interval from one to the next. Presumably in the fall the differences would be more like 29.40 days, to produce the right average.
Feb 21 03:31
Mar 21 18:40 29 days 15 hr 9 min = 29.63 days
Apr 20 10:25 29 days 15 hr 45 min = 29.66 days
May 20 02:11 29 days 15 hr 46 min = 29.66 days
Jun 18 17:30 29 days 15 hr 19 min = 29.64 days
Jul 18 07:59 29 days 14 hr 29 min = 29.60 days
- If you want to calculate phase times accurately, I don't think there's any reasoable way except to numerically simulate the orbits of both bodies in three dimensions. --Anonymous, 01:04 UTC, February 8, 2008, 21 hours 20 minutes past new moon.
Bleaching chemicals[edit]
This is for a sort of literary endeavour, not for any practical purpose. I'm asking on somebody else's behalf, as he hasn't got access at the moment, and he hasn't explained exactly what plot element this is for, so this may sound vague. I think it may be to explain a character being essentially colourless, but I don't know for sure.
Is there any one chemical that could strip the colour out of: hair, skin, leather, cotton, polyester and silk? If not, what chemicals would be suitable for the above materials? (I know what I use on hair, but are there others?)
Thanks in advance. MorganaFiolett (talk) 13:20, 5 February 2008 (UTC)
- Bleach? Bound to make a dent in them, at least as a starting point. Lanfear's Bane | t 15:49, 5 February 2008 (UTC)
- I am now told that all the components are on a living person- i.e. it's one man and his clothes. Apparently he wants his character to get swallowed by a giant monster, get totally bleached on the inside, and then shoot his way out. It's steampunk/sci-fi setting but he likes things to be feasible (rather than cause discussions like the Terminator one from the other day). MorganaFiolett (talk) 16:08, 5 February 2008 (UTC)
Hydrogen peroxide is used on both hair and skin. I'm not sure how compatible it is with all fabrics, however. Cotton's okay, but I don't know about the others off the top of my head. TenOfAllTrades(talk) 15:59, 5 February 2008 (UTC)
- Some dyes will be resistant to most bleaches... However I too would guess that hydrogen peroxide would be a good start - I doubt though that it would turn black into white without serious skin damage.87.102.114.230 (talk) 18:54, 5 February 2008 (UTC)
- I'd concur with peroxide. There are biological mechanisms (example of generation, example of use) which can generate peroxide, and it may be feasible that some sci-fi monster would employ peroxide in digesting food. The only question is amount and concentration. It's highly likely that amounts of peroxide sufficient to turn someone into an albino would be more than enough to kill them. Even if they survive, peroxide at high concentrations causes skin to become tough and non-pliable. That said, any chemical which is able to indiscriminately bleach any sort of dye/material is going to be very reactive by definition, and is also likely to kill someone at effective bleaching concentrations. (Dyes are colored because they contain conjugated double bonds and certain metal ions. Chlorine bleach and peroxide work by oxidizing double bonds and metal ions into colorless forms. Problem is, living cells depend on a range of metal ions and double bonded compounds to survive. Anything indiscriminant enough to oxidize any dye is going to also oxidize vital cellular components.) Peroxide is going to be the mildest/safest one (as cells have ways of dealing with peroxide), and also will be the most biologically feasible one for the monster to make. Note that it doesn't necessarily have to be hydrogen peroxide, as there are other types of peroxides (Organic peroxides like benzoyl peroxide, Ascaridole, Artemisinin, Prostaglandin H2, etc. or peroxy acids like peracetic acid, etc. - no guarantee that any of these examples act as dye bleaching agents, though) - some of which may be less likely to get into cells, avoiding major damage. However, some pigments are within cells, and thus would not be bleached by such non-cell membrane permeable agents. -- 128.104.112.19 (talk) 19:25, 5 February 2008 (UTC)
- Thank you very much for all the responses. I'm the person who asked Morgana for the help. That last response is incredibly helpful. I mainly wanted to know if it was feasible for something to bleach all of those things and still allow someone to survive contact with it. With all of the information I've been given though I might just have to work an explanation for the bleaching into the story.
- I have another question now. Could being essentially submerged completely in an organic peroxide cause hair to grow back white? This character has white hair and I'm basically trying to come up with a dramatic reason for his hair being white. Oh, also, he would be leaping into fresh water almost immediately after he's 'eaten'.83.104.236.154 (talk) 20:39, 5 February 2008 (UTC)
- Grow back white after bleaching? I doubt it. If the person survives the bleaching process, the follicular cells would probably still be alive (have to be for hair to still grow). If the DNA is still intact, the cells would go back to what they were doing before - like bleach blonds, whose brunette hair shows after a month or so if they don't touch up the roots. The only way for hair to grow back white is if the melanocytes in the hair follicle die off - like they do when people get old. There is some thought that stress/trauma can cause hair to turn white, but I see conflicting info on the validity of this perception [3] [4] [5]. There seems there might be a genetic component to stress-induced hair whitening - some people may be prone to having hair turn grey, and stress just speeds up the process. However, if your desire is to have the character's hair turn white eventually (as opposed to having it turn white immediately after being spit out), you could dispense with the bleach, and make up some melanocyte specific poison which kills off the melanocytes, causing all new hair growth after that date to be white. Since hair melanocytes are more prone to death than skin melanocytes (we get grey hair as we age, but not grey skin), he could get just the right dose to kill the hair melanocytes but not the skin ones. Or you could go for the totally white albino (DaVinci Code/Princess Bride anyone?). You could even combine the melanocyte poison with the bleach to get both immediate and long term effects. (Although why a monster has a melanocyte specific poison in their digestive system is anyones guess - though natural organisms have compounds which can used for other purposes: e.g. Sweet Wormwood doesn't get malaria, but it contains Artemisinin (an anti-malarial) and the Madagascar periwinkle contains Vinblastine, a leukemia treatment.) -- 128.104.112.19 (talk) 22:12, 6 February 2008 (UTC)
- While you're thinking bleach, you could think about inducing oculocutaneous albinism – it would need an idea that explains knnocking out a gene or two instead, as with a big spooky case of vitiligo but hey this is sci fi after all... and some kind of radiation always comes in handy. By the way is this character a Jonah? Julia Rossi (talk) 01:20, 6 February 2008 (UTC)
Black Holes[edit]
I was just reading the article on Hawking radiation and it stated that just beyond the event horizon of a black hole, virtual particles are "boosted" into real particles by the gravity of the black hole. How is it possible for virtual particles to become real particles while still observing the law that matter cannot be destroyed or created? Zrs 12 (talk) 15:24, 5 February 2008 (UTC)
- Because there is no law about matter's destruction or creation. Matter and energy are interchangeable, per e=mc² and all that. — Lomn 15:30, 5 February 2008 (UTC)
- I believe he's talking about the conservation of matter. I'm not sure, but I think this law was intended mostly as a chemistry thing, rather than a physics thing. The guy who came up with it, didn't know about fusion, antimatter, etc. 64.236.121.129 (talk) 20:59, 5 February 2008 (UTC)
- Yes, and I should have phrased that better. Conservation of matter should be understood as an everyday approximation (where it is absolutely relevant) but not as a physical law. — Lomn 21:24, 5 February 2008 (UTC)
- I believe he's talking about the conservation of matter. I'm not sure, but I think this law was intended mostly as a chemistry thing, rather than a physics thing. The guy who came up with it, didn't know about fusion, antimatter, etc. 64.236.121.129 (talk) 20:59, 5 February 2008 (UTC)
- One option is that the book is fantasy/speculation and not fact, or at least that the text is not 100% 'true' whatever that is87.102.114.230 (talk) 18:52, 5 February 2008 (UTC)
- I'm not sure it is possible. John Baez says that he's never actually seen Hawking radiation derived from the virtual particle picture. In Hawking's original paper, where this description originated, he says that it's "heuristic only and should not be taken too literally." So if virtual particles don't help you understand black holes, it's probably their fault, not yours. -- BenRG (talk) 19:21, 5 February 2008 (UTC)
Yes, I know matter and energy are inerchagable, and I know that the law of conservation of matter never is correct (matter is converted to energy even in ordinary chemical reactions even though it is a very small amount). So, is it that virtual particles are just energy and the energy added by the gravity of the black hole is enough to boost them into becoming real? Zrs 12 (talk) 22:36, 5 February 2008 (UTC)
- As stated in the article, it is often considered that one of the particles of the virtual pair has negative energy (and thus, negative mass), and this is the one swallowed by the black hole. Under this assumption, what I can't fathom is why the black hole would prefer the negative one (I understand very well that it has to to preserve energy conservation, but that simply doesn't satisfy me). Someguy1221 (talk) 00:34, 6 February 2008 (UTC)
- No, that's not what happens. Assuming it's real (there is no evidence for it), what happens is that a pair of particles (always a pair - one matter, one anti-matter), randomly appear, due to Heisenberg's uncertainty principle, that is, for a very short period of time, matter (AKA energy) is allowed to exist. Now if you do that near a black hole, one of the particles will be closer then the other, that one will be sucked into the black hole, the other might not be - why only one and not both? Because of conservation of momentum. Now comes the tricky part - the particles only "existed", IF they arrived in such a way as to follow all the conservation laws (mass, momentum, quantum numbers, etc). If the particles arrived under any conditions where they don't follow the law - they never existed in the first place! I know, it's confusing. So, it's like they have the ability to exist, from nothing, but only if they go back to being nothing - that's why zero point energy doesn't work - if you try to get energy from nothing, the particles never existed in the first place! In a black hole the energy for the surviving particle is taken from the black hole (presumably the anti-matter particle hits the black hole, and the energy produced from the annihilation is what creates the remaining particle - suppose the matter one fell in, then no annihilation, and the anti-matter one can not exist - since it can't, it never did). Ariel. (talk) 18:41, 8 February 2008 (UTC)
Uranium electron configuration[edit]
I'm reading up on electron configuration. Apart from having trouble keeping the order right (4s before 3d, 5f after 7s...), I can't seem to figure out why the configuration of uranium is the way it is ([Rn] 5f3 6d1 7s2). That makes a full 7s, but why are there electrons in both the 5f and 6d subshells when neither is full nor half-full? Keep in mind that I'm pretty new to this, by the way. ;) Thanks. Aeluwas (talk) 19:23, 5 February 2008 (UTC)
- This is somewhat common (look up some other atoms in the f-block). The 6d orbitals are very close in energy to the 5f orbitals, and so it is very hard to predict where the electrons will fall. It is generally considered that the first 6d electron has a lower energy than the 5f orbitals, so you usually see one electron enter the d orbital before filling in any f orbitals (actinium and neptunium, for example). And due to the closeness of these orbitals in energy, the little things can change this. For example, having the 5f orbitals empty and having a single pair in a 6d-orbital is particularly stable (thorium), while having one electron in each 5f orbital and none in the 6d orbitals is also particularly stable (americium). Someguy1221 (talk) 00:26, 6 February 2008 (UTC)
"Will It Blend" -- what WON'T blend????[edit]
What WON'T blend? —Preceding unsigned comment added by 212.51.122.6 (talk) 19:32, 5 February 2008 (UTC)
- Diamond? ;) -- Aeluwas (talk) 19:35, 5 February 2008 (UTC)
- A decent set of pliers, a good knife, an axeblade, a decent screwdriver...anything made from a good quality tool steel. --Stephan Schulz (talk) 19:38, 5 February 2008 (UTC)
- Antimatter. 206.252.74.48 (talk) 19:54, 5 February 2008 (UTC)
- Chuck Norris. HYENASTE 20:10, 5 February 2008 (UTC)
- Antimatter. 206.252.74.48 (talk) 19:54, 5 February 2008 (UTC)
- A decent set of pliers, a good knife, an axeblade, a decent screwdriver...anything made from a good quality tool steel. --Stephan Schulz (talk) 19:38, 5 February 2008 (UTC)
Anything brittle 64.236.121.129 (talk) 20:44, 5 February 2008 (UTC)
- See Will it blend for source of meme. --LarryMac | Talk 21:09, 5 February 2008 (UTC)
- Peanut brittle will probably blend deliciously :D\=< (talk) 22:31, 5 February 2008 (UTC)
Stop this! This is getting too silly! -- MacAddct 1984 (talk • contribs) 22:42, 5 February 2008 (UTC) A crowbar didn't blend... they almost tried it. Zrs 12 (talk) 01:02, 6 February 2008 (UTC)
fioracet[edit]
Is fioracet addictive? —Preceding unsigned comment added by 70.128.99.57 (talk) 20:08, 5 February 2008 (UTC)
- Ooh, that's a medical question. You should consult a qualified doctor about that. HYENASTE 20:11, 5 February 2008 (UTC)
- Agreed, but feel free to consult our article on Fioricet, which does list addiction as a potential side-effect. Bovlb (talk) 21:23, 5 February 2008 (UTC)
- I disagree, this is a straightforward question with a straightforward answer, which does not constitute advice or subjective opinion. The answer is yes, fioracet can be addictive, on account of the Butalbital contained within. Tuckerekcut (talk) 03:52, 6 February 2008 (UTC)
- Agreed, but feel free to consult our article on Fioricet, which does list addiction as a potential side-effect. Bovlb (talk) 21:23, 5 February 2008 (UTC)
Why does radioactive fallout occur in fission bombs but not pure fusion bombs?[edit]
^Topic. 64.236.121.129 (talk) 20:45, 5 February 2008 (UTC)
- There's no such thing as a pure fusion bomb. All fusion bombs have a fission core and most (all, maybe), certainly all those designed using the Teller-Ulam design, also have a uranium "sparkplug" which fissions too. Nuclear fission creates nasty radionucleides because you're smashing a giant nucleus into two two pretty large radioisotopes, whereas nuclear fusion has to build nucleii up from basic ingredients (and in general small simple nucleii are stable and big fancy ones aren't, and unstable nucleii fall apart(==radioactivity)). -- Finlay McWalter | Talk 21:07, 5 February 2008 (UTC)
- Yes, I know it doesn't exist, but a pure fusion bomb is entirely possible. 64.236.121.129 (talk) 22:05, 5 February 2008 (UTC)
- It's not physically impossible (see, for example, the sun). We just haven't the foggiest idea of how to make it work on a human scale and with our existing technology. --24.147.69.31 (talk) 22:37, 5 February 2008 (UTC)
- The reaction that provides the Sun's energy, the proton-proton chain, is too slow for a bomb, I think. Good thing too, or the Sun wouldn't last long. --Trovatore (talk) 19:30, 6 February 2008 (UTC)
- It's not physically impossible (see, for example, the sun). We just haven't the foggiest idea of how to make it work on a human scale and with our existing technology. --24.147.69.31 (talk) 22:37, 5 February 2008 (UTC)
- Fallout is largely caused by heavy fission products and dust/debris that is made radioactive by these products. The heavy particles are lifted up with the heat of the fireball and then later cool and fall back down to earth again. I'm not sure whether you'd get the same effect with the radioactive by-products of fusion reactions, but in any case I don't think any of the by-products of fusion reactions are going to be anything as radioactive as fission products and certainly not as heavy. You get a lot of neutrons from fusion reactions so I could imagine you irradiating a lot of dust, but I don't know offhand how that would compare with a fission weapon, except to say that it is clear that any large fusion bomb would have much, much less fallout that a comparative bomb using fission in any form (compare the fallout difference between the "clean" Tsar Bomba and the untested "dirty" version—it's quite significant, owing to the removal of the final fission stage). I'm sure some cunning engineer or physicist could run the numbers, though. --24.147.69.31 (talk) 22:37, 5 February 2008 (UTC)
- I don't understand. So you split plutonium into... what? And you fuse Hydrogen into Helium I think? So where does the radiation fit in? 64.236.121.129 (talk) 19:21, 6 February 2008 (UTC)
- During nuclear fission, you get smaller nuclei. Some of those smaller nuclei are alpha particles. Some other particles released include beta rays. And a lot of energy may be released, which is emitted in the form of gamma rays. DMacks (talk) 19:35, 6 February 2008 (UTC)
- Note that fallout itself isn't directly any of these, but rather the other nuclei (fission products) that are themselves unstable, releasing alpha, beta, and/or gamma radiation over a longer period. As for fusion, it's not a simple case of 4H=>He, but rather the fusing of heavy isotopes, one of which is already radioactive and the process of which can generate radioactive non-helium elements. The latter link also explains part of the problem of a "pure fusion" bomb, by the way. — Lomn 20:12, 6 February 2008 (UTC)
- Right. After nuclear fission you get a handful of tiny particles and two BIG OL' unstable particles. It's pretty much random which ones you get—it's pretty much an even distribution of possibilities—but they're all unstable and violently radioactive (very short half-lives = very energetic, very dangerous). --24.147.69.31 (talk) 23:13, 6 February 2008 (UTC)
- Well, there's a reason for that. The more protons you try to pack into a nucleus, the higher the ratio of neutrons to protons you need to make it stable or near-stable. The nuclides used in fission weapons are near-stable for obvious reasons (half lives in the tens of thousands of years to billions of years) so they have a lot of neutrons per proton. When you split a nucleus, each piece has around the same ratio of neutrons to protons -- but that is now too high for these smaller nuclei, so they decay until they reach a more stable configuration. --Trovatore (talk) 01:33, 7 February 2008 (UTC)
- Right. After nuclear fission you get a handful of tiny particles and two BIG OL' unstable particles. It's pretty much random which ones you get—it's pretty much an even distribution of possibilities—but they're all unstable and violently radioactive (very short half-lives = very energetic, very dangerous). --24.147.69.31 (talk) 23:13, 6 February 2008 (UTC)
- Note that fallout itself isn't directly any of these, but rather the other nuclei (fission products) that are themselves unstable, releasing alpha, beta, and/or gamma radiation over a longer period. As for fusion, it's not a simple case of 4H=>He, but rather the fusing of heavy isotopes, one of which is already radioactive and the process of which can generate radioactive non-helium elements. The latter link also explains part of the problem of a "pure fusion" bomb, by the way. — Lomn 20:12, 6 February 2008 (UTC)
- During nuclear fission, you get smaller nuclei. Some of those smaller nuclei are alpha particles. Some other particles released include beta rays. And a lot of energy may be released, which is emitted in the form of gamma rays. DMacks (talk) 19:35, 6 February 2008 (UTC)
- I don't understand. So you split plutonium into... what? And you fuse Hydrogen into Helium I think? So where does the radiation fit in? 64.236.121.129 (talk) 19:21, 6 February 2008 (UTC)
What happens if you feed a cow a cookie?[edit]
I was asked this rather strange question, and I didn't know where else to ask. Anyone know? 64.236.121.129 (talk) 20:56, 5 February 2008 (UTC)
- This is original research, but I'm going to say that it would digest it (unless it is dead, of course). 206.252.74.48 (talk) 21:03, 5 February 2008 (UTC)
- Presumably you needn't worry about chocolate chips since chocolate milk cows drink it fine :D\=< (talk) 22:30, 5 February 2008 (UTC)
- Do you think it would want a glass of milk? -- MacAddct 1984 (talk • contribs) 22:31, 5 February 2008 (UTC)
- Excellent followup, MacAddct1984! I had the same thought and then saw your response. Thomprod (talk) 03:51, 6 February 2008 (UTC)
- Same here. Ilikefood (talk) 22:16, 6 February 2008 (UTC)
- Cookies ARE included in cattle feed sometimes (sort of). Animal nutritionists are always looking for cheap feed ingredients to include in feed to give the animal all the nutrients it needs at the least possible cost. One such ingredient is "cookie meal" which is primarily crumbs and broken bits from industrial bakeries. ike9898 (talk) 15:09, 7 February 2008 (UTC)
- Same here. Ilikefood (talk) 22:16, 6 February 2008 (UTC)
- Excellent followup, MacAddct1984! I had the same thought and then saw your response. Thomprod (talk) 03:51, 6 February 2008 (UTC)
- Do you think it would want a glass of milk? -- MacAddct 1984 (talk • contribs) 22:31, 5 February 2008 (UTC)
Within 24 hours the cow will convert the cookie into a "cow pasture patty" aka cow poop.
Cloning of the PCR product[edit]
From Bisulphite sequencing: This technique required cloning of the PCR product prior to sequencing for adequate sensitivity, and therefore was a very labour-intensive method unsuitable for higher throughput. "Cloning of the PCR product"? Since PCR is an amplification procedure, how does this make sense? I see it like saying that the clean T shirts needed to be cleaned. ----Seans Potato Business 21:13, 5 February 2008 (UTC)
- I really don't know much about this, but the sentence you quoted only makes sense by reading "cloning" as you would in the pre-PCR era, i.e. putting a gene into a vector. --NorwegianBlue talk 21:58, 5 February 2008 (UTC)
Trees[edit]
How much does one Red Oak tree help our environment? Assuming they are planted correctly and taken care of properly, how much would one tree do to combat global warming. And can I assume that planting 8 red oaks will combat global warming 8 time as efficiently as one? Thanks, schyler (talk) 21:38, 5 February 2008 (UTC)
8 will not not combat it 8 times more than one, since one tree will grow one way and 7 additional trees may all grow larger or smaller with a potentially greater or lesser than 1 times 8 threashhold. it will be true that 8 average trees will help 8 times more than 1 average tree. that is on average. —Preceding unsigned comment added by Boomgaylove (talk • contribs) 05:37, 6 February 2008 (UTC)
- Planting trees does little for global warming, what counts is growing trees, and bigger trees tend to grow faster than smaller trees. Claims for carbon credits for planting are a fraud, if it does not support the growth also. Graeme Bartlett (talk) 08:55, 7 February 2008 (UTC)
Why is there this much matter?[edit]
Why is there this much matter instead of more, or less? Also more specifically, the Big Bang theory supposes a singularity at the beginning of our effective timeline..
- If it's a singularity, again how do we have this amount of matter instead of more or less? How can one singularity be more massive than another?
- How would it escape its own gravity well? Wouldn't the universe explode or someth- oh.
- Does the Big Bang model say anything about why itself happened, or does it sort of awkwardly cough and change the subject?
:D\=< (talk) 22:25, 5 February 2008 (UTC)
- A lot of those questions are not yet answered or, sadly, unanswerable. I don't think it will ever be possible to know what happened before planck time. -- MacAddct 1984 (talk • contribs) 22:38, 5 February 2008 (UTC)
- This is just what I heard on a show on the science chanel and may not have understood (so please correct any information that is incorrect); M theory says that the membranes surrounding universes touch against each other sometimes. This is what supposedly creates singularities. (This is of corse accepting parallel universes). Yet, this begs the question, "How would the first universe have come into existence?", and furthermore it poses the question, "How can these collisions create matter?". So, obviously there are a lot of gaps in the current theories. I'm not sure that your questions are currently answerable. Zrs 12 (talk) 22:54, 5 February 2008 (UTC)
- Membranes between universes? This is the science desk, maybe someone can identify which specific psychoactive drug may have caused that theory to be spawned --:D\=< (talk) 23:56, 5 February 2008 (UTC)
- Haha. Yeah, universes enclosed in 4-dimensional membranes. Take a look at these links. They maybe helpful. m-theory, superstring theory, quantum gravity, string theory, list of string theory topics#Branes, list of string theory topics#Particles and fields, list of string theory topics#String duality, list of string theory topics#String theory, especially, m-theory#Big Bang Theory Zrs 12 (talk) 00:31, 6 February 2008 (UTC)
- Membranes between universes? This is the science desk, maybe someone can identify which specific psychoactive drug may have caused that theory to be spawned --:D\=< (talk) 23:56, 5 February 2008 (UTC)
- Some things in this area are very well understood; for example, the fact that the ordinary matter in the universe is about 75% hydrogen and 25% helium is explained by big bang nucleosynthesis, and the abundances of other elements by stellar nucleosynthesis and supernova nucleosynthesis, all of which are understood in amazing detail (but not by me). The fact that the total mass-energy density is almost exactly the critical density is surely not an accident, and one of the big successes of inflationary cosmology was explaining this is a reasonably natural way (though it's still far from clear that inflationary cosmology itself is correct). No one knows the nature of the dark matter or dark energy, or the reason for the ratio of ~4% ordinary matter, ~22% dark matter and ~76% dark energy. The matter density was of course higher in the past, and will be lower in the future, so at some level the density in the present era can only sensibly be explained anthropically (though I hate to say that since the word "anthropic" is being attached to so much nonsense these days).
- Big Bang cosmology does not say there's a singularity. There's a singularity in the mathematical model, but nobody thinks that part of the model has any physical significance. Take a look at Age of the universe#Explanation, particularly the second paragraph. Big Bang cosmology, practically by definition, doesn't explain its own initial conditions. There are lots of speculative ideas, mostly variations on inflation, but until there's a way to test them there's not much that really qualifies as physics. The M-theory and brane-world stuff has the same problem. The great majority of ideas like this are going to turn out wrong. They get reported on because they sound cool, not because they have much support in the physics community. -- BenRG (talk) 15:04, 6 February 2008 (UTC)
Converting a linear force to angular momentum[edit]
What is the formula, if there is one, for converting a linear force to an angular momentum when two objects collide? In other words, how do I take into consideration that the two objects will generate torque from colliding with each other? --PSv255 (talk) 22:47, 5 February 2008 (UTC)
- First of all, taking a look at Momentum, and Angular Momentum, and Conservation of momentum might help. I'm not quite sure what you mean by "generate torque", but keep in mind that the total angular momentum of the system does not change during the collision; whatever angular momentum there was before, is still there afterwards. Same goes for linear momentum. In terms of "converting" linear momentum to angular momentum (which is a bit imprecise, since they are different quantities), angular momentum , where is linear momentum, and is the vector between a reference point and the particle; note that in general, angular momentum is only defined "about" a point - it is reference-point dependent (but always conserved, regardless of reference point). Hope this helps. --Bmk (talk) 03:31, 6 February 2008 (UTC)




