Kelvin: Difference between revisions
Em3rgent0rdr (talk | contribs) →Lord Kelvin: no quotes around "absolute", just hyperlink to absolute scale instead |
Em3rgent0rdr (talk | contribs) putting "exact" before temperature here, since is a condition for the result to be exact. |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 24: | Line 24: | ||
}} |
}} |
||
The '''kelvin''', symbol '''K''', is the [[base unit of measurement]] for [[temperature]] in the [[International System of Units]] (SI).<ref name="MEP for kelvin 2019"/><ref name="SI Brochure 9"/><ref name="BIPM web page for kelvin"/><ref name="NIST SI redefinition"/> It is named after |
The '''kelvin''', symbol '''K''', is the [[base unit of measurement]] for [[temperature]] in the [[International System of Units]] (SI).<ref name="MEP for kelvin 2019"/><ref name="SI Brochure 9"/><ref name="BIPM web page for kelvin"/><ref name="NIST SI redefinition"/> It is named after the 19{{Sup|th}} century British scientist [[William Thomson, 1st Baron Kelvin]]<ref name="nist intro"/> who proposed an [[absolute scale|absolute]] [[temperature scale]] (now named the '''Kelvin scale''') which assigned [[zero]] for the coldest possible temperature (now called [[absolute zero]] and known to be approximately -273.15 °C) but which also kept the same temperature increment used in the well-established [[Celsius]] scale (symbol °C). |
||
A consequence of these conditions is that 273.15 is added to numerical value of a temperature expressed in Celsius to express it in kelvin. Thus the approximate [[melting point]] of [[ice]], which is fixed at 0 °C in the Celsius scale, instead has the value 273.15 K in the Kelvin scale.<ref name="MEP for kelvin 2019" /><ref name="nist intro" /> Although the Celsius, [[Fahrenheit]], and [[Rankine scale|Rankine]] scales are now defined in terms of the Kelvin scale, this relationship remains.<ref name="SI Brochure 9"/><ref name="Busting Myths about the Metric System"/><ref name="NIST HB44 Appendix C"/> |
|||
Measurement improvements gradually provided more precise values for the melting point and even more precise values for water's similar [[triple point]] temperature (which consequently became [[General Conference on Weights and Measures#CGPM meetings|the kelvin's reference in 1954]]). But because uncertainty still remains in the triple point measurement, the [[2019 redefinition of the SI base units]] now defines the kelvin by setting the [[Boltzmann constant]] to ''exactly'' {{physconst|k|ref=no|symbol=no|unit=no}} [[joules]] per kelvin, thereby removing any measurement uncertainty from the Boltzmann constant.<ref name="SI Brochure 9" /> [[KT (energy)|Multiplying the Boltzmann constant by an exact temperature]] now yields an exact energy, so every 1 K change of [[thermodynamic temperature]] corresponds to a [[thermal energy]] change of exactly {{val|1.380649|e=−23|u=J}}. |
|||
== History == |
== History == |
||
Revision as of 00:35, 3 May 2024
| kelvin | |
|---|---|
 Equivalent temperatures in kelvin (K), Celsius (°C), and Fahrenheit (°F) | |
| General information | |
| Unit system | SI |
| Unit of | temperature |
| Symbol | K |
| Named after | William Thomson, 1st Baron Kelvin |
| 2019 definition | kB = 1.380649×10−23 J/K |
| Conversions | |
| x K in ... | ... corresponds to ... |
| Celsius | (x − 273.15) °C |
| Fahrenheit | (1.8 x − 459.67) °F |
| Rankine | 1.8 x °Ra |
The kelvin, symbol K, is the base unit of measurement for temperature in the International System of Units (SI).[1][2][3][4] It is named after the 19th century British scientist William Thomson, 1st Baron Kelvin[5] who proposed an absolute temperature scale (now named the Kelvin scale) which assigned zero for the coldest possible temperature (now called absolute zero and known to be approximately -273.15 °C) but which also kept the same temperature increment used in the well-established Celsius scale (symbol °C).
A consequence of these conditions is that 273.15 is added to numerical value of a temperature expressed in Celsius to express it in kelvin. Thus the approximate melting point of ice, which is fixed at 0 °C in the Celsius scale, instead has the value 273.15 K in the Kelvin scale.[1][5] Although the Celsius, Fahrenheit, and Rankine scales are now defined in terms of the Kelvin scale, this relationship remains.[2][6][7]
Measurement improvements gradually provided more precise values for the melting point and even more precise values for water's similar triple point temperature (which consequently became the kelvin's reference in 1954). But because uncertainty still remains in the triple point measurement, the 2019 redefinition of the SI base units now defines the kelvin by setting the Boltzmann constant to exactly 1.380649×10−23 joules per kelvin, thereby removing any measurement uncertainty from the Boltzmann constant.[2] Multiplying the Boltzmann constant by an exact temperature now yields an exact energy, so every 1 K change of thermodynamic temperature corresponds to a thermal energy change of exactly 1.380649×10−23 J.
History
Precursors

During the 18th century, multiple temperature scales were developed,[8] notably Fahrenheit and centigrade (later Celsius). These scales predated much of the modern science of thermodynamics, including atomic theory and the kinetic theory of gases which underpin the concept of absolute zero. Instead, they chose defining points within the range of human experience that could be reproduced easily and with reasonable accuracy, but lacked any deep significance in thermal physics. In the case of the Celsius scale (and the long since defunct Newton scale and Réaumur scale) the melting point of water served as such a starting point, with Celsius being defined (from the 1740s to the 1940s) by calibrating a thermometer such that:
- Water's freezing point is 0 °C.
- Water's boiling point is 100 °C.
This definition assumes pure water at a specific pressure chosen to approximate the natural air pressure at sea level. Thus, an increment of 1 °C equals 1/100 of the temperature difference between the melting and boiling points. The same temperature interval was later used for the Kelvin scale.
Charles's law
From 1787 to 1802, it was determined by Jacques Charles (unpublished), John Dalton,[9][10] and Joseph Louis Gay-Lussac[11] that, at constant pressure, ideal gases expanded or contracted their volume linearly (Charles's law) by about 1/273 parts per degree Celsius of temperature's change up or down, between 0 °C and 100 °C. This suggested that the volume of a gas cooled at about −273 °C would reach zero.
Lord Kelvin

In 1848, William Thomson, who was later ennobled as Lord Kelvin, published a paper On an Absolute Thermometric Scale.[12][13][14] Using the soon-to-be-disused caloric theory, he proposed an absolute scale based on the following parameters:
- The melting point of water is 0 degrees.
- The boiling point of water is 100 degrees.
"The arbitrary points which coincide on the two scales are 0° and 100°"
- Any two heat engines whose heat source and heat sink are both separated by the same number of degrees will, per Carnot's theorem, be capable of producing the same amount of mechanical work per unit of "caloric" passing through.
"The characteristic property of the scale which I now propose is, that all degrees have the same value; that is, that a unit of heat descending from a body A at the temperature T° of this scale, to a body B at the temperature (T − 1)°, would give out the same mechanical effect, whatever be the number T. This may justly be termed an absolute scale, since its characteristic is quite independent of the physical properties of any specific substance."
As Carnot's theorem is understood in modern thermodynamics to simply describe the maximum efficiency with which thermal energy can be converted to mechanical energy and the predicted maximum efficiency is a function of the ratio between the absolute temperatures of the heat source and heat sink:
- Efficiency ≤ 1 − absolute temperate of heat sink/absolute temperature of heat source
It follows that increments of equal numbers of degrees on this scale must always represent equal proportional increases in absolute temperature.
The numerical value of an absolute temperature, T, on the 1848 scale is related to the absolute temperature of the melting point of water, Tmelt, and the absolute temperature of the boiling point of water, Tboil, by
- T (1848 scale) =
On this scale, an increase of 222 degrees always means an approximate doubling of absolute temperature regardless of the starting temperature.
In a footnote Thomson calculated that "infinite cold" (absolute zero, which would have a numerical value of negative infinity on this scale) was equivalent to −273 °C using the air thermometers of the time. This value of "−273" was the negative reciprocal of 0.00366—the accepted coefficient of thermal expansion of an ideal gas per degree Celsius relative to the ice point, giving a remarkable consistency to the currently accepted value.[15]
Within a decade, Thomson had abandoned caloric theory and superseded the 1848 scale with a new one[13][16] based on the 2 features that would characterise all future versions of the Kelvin scale:
- Absolute zero is the null point.
- Increments have the same magnitude as they do in the Celsius scale.
In 1892, Thomson was awarded the noble title 1st Baron Kelvin of Largs, or more succinctly Lord Kelvin. This name was a reference to the River Kelvin which flows through the grounds of Glasgow University.
In the early decades of the 20th century, the Kelvin scale was often called the "absolute Celsius" scale, indicating Celsius degrees counted from absolute zero rather than the freezing point of water, and using the same symbol for regular Celsius degrees, °C.[a]
Triple point standard

In 1873, William Thomson's older brother James coined the term triple point[17] to describe the combination of temperature and pressure at which the solid, liquid, and gas phases of a substance were capable of coexisting in thermodynamic equilibrium. While any two phases could coexist along a range of temperature-pressure combinations (e.g. the boiling point of water can be affected quite dramatically by raising or lowering the pressure), the triple point condition for a given substance can occur only at a single pressure and only at a single temperature. By the 1940s, the triple point of water had been experimentally measured to be about 0.6% of standard atmospheric pressure and very close to 0.01 °C per the historical definition of Celsius then in use.
In 1948, the Celsius scale was recalibrated by assigning the triple point temperature of water the value of 0.01 °C exactly[18] and allowing the melting point at standard atmospheric pressure to have an empirically determined value (and the actual melting point at ambient pressure to have a fluctuating value) close to 0 °C. This was justified on the grounds that the triple point was judged to give a more accurately reproducible reference temperature than the melting point.[19] The triple point could be measured with ±0.0001 °C accuracy, while the melting point just to ±0.001 °C.[18]
In 1954, with absolute zero having been experimentally determined to be about −273.15 °C per the definition of °C then in use, Resolution 3 of the 10th General Conference on Weights and Measures (CGPM) introduced a new internationally standardized Kelvin scale which defined the triple point as exactly 273.15 + 0.01 = 273.16 degrees Kelvin.[20][21]
In 1967/1968, Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature "kelvin", symbol K, replacing "degree Kelvin", symbol °K.[22][23][24] The 13th CGPM also held in Resolution 4 that "The kelvin, unit of thermodynamic temperature, is equal to the fraction 1/273.16 of the thermodynamic temperature of the triple point of water."[4][25][26]
After the 1983 redefinition of the metre, this left the kelvin, the second, and the kilogram as the only SI units not defined with reference to any other unit.
In 2005, noting that the triple point could be influenced by the isotopic ratio of the hydrogen and oxygen making up a water sample and that this was "now one of the major sources of the observed variability between different realizations of the water triple point", the International Committee for Weights and Measures (CIPM), a committee of the CGPM, affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the kelvin would refer to water having the isotopic composition specified for Vienna Standard Mean Ocean Water.[4][27][28]
2019 redefinition

In 2005, the CIPM began a programme to redefine the kelvin (along with the other SI units) using a more experimentally rigorous method. In particular, the committee proposed redefining the kelvin such that the Boltzmann constant takes the exact value 1.3806505×10−23 J/K.[29] The committee had hoped that the program would be completed in time for its adoption by the CGPM at its 2011 meeting, but at the 2011 meeting the decision was postponed to the 2014 meeting when it would be considered as part of a larger program.[30]
The redefinition was further postponed in 2014, pending more accurate measurements of the Boltzmann constant in terms of the current definition,[31] but was finally adopted at the 26th CGPM in late 2018, with a value of k = 1.380649×10−23 J⋅K−1.[32][29][1][2][4][33]
For scientific purposes, the main advantage is that this allows measurements at very low and very high temperatures to be made more accurately, as the techniques used depend on the Boltzmann constant. It also has the philosophical advantage of being independent of any particular substance. The unit J/K is equal to kg⋅m2⋅s−2⋅K−1, where the kilogram, metre and second are defined in terms of the Planck constant, the speed of light, and the duration of the caesium-133 ground-state hyperfine transition respectively.[2] Thus, this definition depends only on universal constants, and not on any physical artifacts as practiced previously. The challenge was to avoid degrading the accuracy of measurements close to the triple point.
For practical purposes, the redefinition was unnoticed; enough digits were used for the Boltzmann constant to ensure that 273.16 K has enough significant digits to contain the uncertainty of water's triple point[34] and water will still freeze at 0 °C[35] to a high degree of precision. But before the redefinition, the triple point of water was exact and the Boltzmann constant had a measured value of 1.38064903(51)×10−23 J/K, with a relative standard uncertainty of 3.7×10−7.[34] Afterward, the Boltzmann constant is exact and the uncertainty is transferred to the triple point of water, which is now 273.1600(1) K.[b]
The new definition officially came into force on 20 May 2019, the 144th anniversary of the Metre Convention.[33][1][2][4]
Practical uses
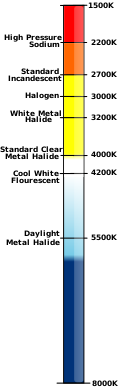
Colour temperature
The kelvin is often used as a measure of the colour temperature of light sources. Colour temperature is based upon the principle that a black body radiator emits light with a frequency distribution characteristic of its temperature. Black bodies at temperatures below about 4000 K appear reddish, whereas those above about 7500 K appear bluish. Colour temperature is important in the fields of image projection and photography, where a colour temperature of approximately 5600 K is required to match "daylight" film emulsions.
In astronomy, the stellar classification of stars and their place on the Hertzsprung–Russell diagram are based, in part, upon their surface temperature, known as effective temperature. The photosphere of the Sun, for instance, has an effective temperature of 5772 K [1][2][3][4] as adopted by IAU 2015 Resolution B3.
Digital cameras and photographic software often use colour temperature in K in edit and setup menus. The simple guide is that higher colour temperature produces an image with enhanced white and blue hues. The reduction in colour temperature produces an image more dominated by reddish, "warmer" colours.
Kelvin as a unit of noise temperature
For electronics, the kelvin is used as an indicator of how noisy a circuit is in relation to an ultimate noise floor, i.e. the noise temperature. The so-called Johnson–Nyquist noise of discrete resistors and capacitors is a type of thermal noise derived from the Boltzmann constant and can be used to determine the noise temperature of a circuit using the Friis formulas for noise.
Derived units and SI multiples
The only SI derived unit with a special name derived from the kelvin is the degree Celsius. Like other SI units, the kelvin can also be modified by adding a metric prefix that multiplies it by a power of 10:
| Submultiples | Multiples | ||||
|---|---|---|---|---|---|
| Value | SI symbol | Name | Value | SI symbol | Name |
| 10−1 K | dK | decikelvin | 101 K | daK | decakelvin |
| 10−2 K | cK | centikelvin | 102 K | hK | hectokelvin |
| 10−3 K | mK | millikelvin | 103 K | kK | kilokelvin |
| 10−6 K | μK | microkelvin | 106 K | MK | megakelvin |
| 10−9 K | nK | nanokelvin | 109 K | GK | gigakelvin |
| 10−12 K | pK | picokelvin | 1012 K | TK | terakelvin |
| 10−15 K | fK | femtokelvin | 1015 K | PK | petakelvin |
| 10−18 K | aK | attokelvin | 1018 K | EK | exakelvin |
| 10−21 K | zK | zeptokelvin | 1021 K | ZK | zettakelvin |
| 10−24 K | yK | yoctokelvin | 1024 K | YK | yottakelvin |
| 10−27 K | rK | rontokelvin | 1027 K | RK | ronnakelvin |
| 10−30 K | qK | quectokelvin | 1030 K | QK | quettakelvin |
Orthography
According to SI convention, the kelvin is never referred to nor written as a degree. The word "kelvin" is not capitalized when used as a unit. It may be in plural form as appropriate (for example, "it is 283 kelvins outside", as for "it is 50 degrees Fahrenheit" and "10 degrees Celsius").[37][5][38][39] The unit's symbol K is a capital letter,[22] per the SI convention to capitalize symbols of units derived from the name of a person.[40] It is common convention to capitalize Kelvin when referring to Lord Kelvin[5] or the Kelvin scale.[41]
The unit symbol K is encoded in Unicode at code point U+212A K KELVIN SIGN. However, this is a compatibility character provided for compatibility with legacy encodings. The Unicode standard recommends using U+004B K LATIN CAPITAL LETTER K instead; that is, a normal capital K. "Three letterlike symbols have been given canonical equivalence to regular letters: U+2126 Ω OHM SIGN, U+212A K KELVIN SIGN, and U+212B Å ANGSTROM SIGN. In all three instances, the regular letter should be used."[42]
See also
- Outline of metrology and measurement
- Comparison of temperature scales
- International Temperature Scale of 1990
- Negative temperature
Notes
References
- ^ a b c d BIPM (2019-05-20). "Mise en pratique for the definition of the kelvin in the SI". BIPM.org. Retrieved 2022-02-18.
- ^ a b c d e f "SI Brochure: The International System of Units (SI) – 9th edition (updated in 2022)". BIPM. Retrieved 2022-09-07.
- ^ "SI base unit: kelvin (K)". BIPM. Retrieved 2022-03-05.
- ^ a b c d e "A Turning Point for Humanity: Redefining the World's Measurement System". NIST. 2018-05-12. Retrieved 2022-02-21.
- ^ a b c d "Kelvin: Introduction". NIST. 2018-05-14. Retrieved 2022-09-02.
- ^ Benham, Elizabeth (2020-10-06). "Busting Myths about the Metric System". NIST. Taking Measure (official blog of the NIST). Retrieved 2022-02-21.
- ^ "Handbook 44 – 2022 – Appendix C – General Tables of Units of Measurement" (PDF). nist.gov. NIST. Retrieved 2022-02-21.
- ^ "Kelvin: History". NIST. 2018-05-14. Retrieved 2022-02-21.
- ^ Dalton, John (1801). "Essay II. On the force of steam or vapour from water and various other liquids, both in vacuum and in air". Memoirs of the Literary and Philosophical Society of Manchester. 5 part 2: 550–574.
- ^ Dalton, John (1801). "Essay IV. On the expansion of elastic fluids by heat". Memoirs of the Literary and Philosophical Society of Manchester. 5 part 2: 595–602.
- ^ Gay-Lussac, Joseph Louis (1802), "Recherches sur la dilatation des gaz et des vapeurs", Annales de Chimie, XLIII: 137. English translation (extract).
- ^ Thomson, William. "On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations". zapatopi.net. Philosophical Magazine. Retrieved 2022-02-21.
- ^ a b Thomson, William. "On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations (1881 reprint)" (PDF). Philosophical Magazine. Retrieved 2022-02-21.
- ^ Kelvin, William (October 1848). "On an Absolute Thermometric Scale". Philosophical Magazine. Archived from the original on 2008-02-01. Retrieved 2008-02-06.
- ^ Thomson, William. "On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations (1881 reprint)" (PDF). Philosophical Magazine. Retrieved 2022-02-21.
If we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/·366, if ·366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low
- ^ Thomson, William. "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule's equivalent of a Thermal Unit, and M. Regnault's Observations on Steam (Excerpts)". Zapatopi.net. Transactions of the Royal Society of Edinburgh and Philosophical Magazine. Retrieved 2022-02-21.
- ^ Thomson, James (1873). "A quantitative investigation of certain relations between the gaseous, the liquid, and the solid states of water-substance". Proceedings of the Royal Society of London. 22: 28. Bibcode:1873RSPS...22...27T. ISSN 0370-1662.
and consequently that the three curves would meet or cross each other in one point, which I have called the triple point.
- ^ a b Swinton, F. L. (September 1967). "The triplet point of water". Journal of Chemical Education. 44 (9): 541. Bibcode:1967JChEd..44..541S. doi:10.1021/ed044p541. ISSN 0021-9584.
- ^ "Resolution 3 of the 9th CGPM (1948)". BIPM. Retrieved 2022-02-21.
- ^ "Resolution 3 of the 10th CGPM (1954)". BIPM. Retrieved 2022-02-21.
- ^ "Resolution 3: Definition of the thermodynamic temperature scale". Resolutions of the 10th CGPM. Bureau International des Poids et Mesures. 1954. Archived from the original on 2007-06-23. Retrieved 2008-02-06.
- ^ a b "Resolution 3 of the 13th CGPM (1967)". BIPM. Retrieved 2022-02-21.
- ^ "Resolution 3: SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. Archived from the original on 2007-04-21. Retrieved 2008-02-06.
- ^ Westphal, Wilhelm Heinrich (1952). "Nox, Dunkelleuchtdichte, Skot". In Westphal, Wilhelm H. (ed.). Physikalisches Wörterbuch (in German) (1 ed.). Berlin / Göttingen / Heidelberg, Germany: Springer-Verlag OHG. pp. 125, 271, 389. doi:10.1007/978-3-662-12706-3. ISBN 978-3-662-12707-0. Retrieved 2023-03-16. pp. 271, 389:
Dunkelleuchtdichte. […] Unter Zugrundelegung dieser Empfindlichkeitskurve hat man 1940 in Deutschland die Dunkelleuchtdichte mit der Einheit Skot (sk) so festgesetzt, daß bei einem Licht der Farbtemperatur 2360 °K 1 sk = 10−3 asb gilt. 1948 ist von der Internationalen Beleuchtungskommission (IBK) die Bezugstemperatur auf 2046 °K, die Erstarrungstemperatur des Platins, festgesetzt worden. Die Bezeichnung Skot wurde von der IBK nicht übernommen, dafür soll "skotopisches Stilb" gesagt werden. Als höchstzulässiger Grenzwert für die Dunkelleuchtdichte ist in Deutschland 10 Skot festgesetzt worden, um eine Verwendung der Dunkelleuchtdichte im Gebiet des gemischten Zapfen- und Stäbchensehens zu vermeiden, da in diesem Bereich die photometrischen Maßgrößen wegen der allmählich gleitenden Augenempfindlichkeitskurve ihren Sinn verlieren. [...] Skot, abgek[ürzt] sk, Einheit für die Dunkelleuchtdichte, welche für zahlenmäßige Angaben und zum Anschluß der Dunkelleuchtdichte an die normale Leuchtdichte 1940 von der Deutschen Lichttechnischen Gesellschaft geschaffen wurde. Für diesen Anschluß wurde die Strahlung des schwarzen Körpers bei T = 2360 °K, d.h. eine Strahlung der Farbtemperatur T1 = 2360 °K vereinbart. Eine Lichtquelle strahlt mit der Dunkelleuchtdichte 1 sk, wenn sie photometrisch gleich einer Strahlung der Farbtemperatur T2 = 2360 °K und der Leuchtdichte von 10−3 asb (Apostilb) ist. Bei der Farbtemperatur T1 = 2360 °K gilt also die Relation: 1 sk = 10−3 asb = 10−7/π sb.
- ^ "Resolution 4 of the 13th CGPM (1967)". BIPM. Retrieved 2022-02-21.
- ^ "Resolution 4: Definition of the SI unit of thermodynamic temperature (kelvin)". Resolutions of the 13th CGPM. Bureau International des Poids et Mesures. 1967. Archived from the original on 2007-06-15. Retrieved 2008-02-06.
- ^ "Resolution 10 of the 23rd CGPM (2007)". BIPM. Retrieved 2022-02-21.
- ^ "Unit of thermodynamic temperature (kelvin)". SI Brochure, 8th edition. Bureau International des Poids et Mesures. 1967. "Section 2.1.1.5". Archived from the original on 2007-09-26. Retrieved 2008-02-06.
- ^ a b Ian Mills (2010-09-29). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). BIPM. CCU. Archived from the original (PDF) on 2011-01-10. Retrieved 2011-01-01.
- ^ "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. 2011-10-23. Archived from the original (PDF) on 2012-02-09. Retrieved 2011-10-25.
- ^
Wood, B. (3–4 November 2014). "Report on the Meeting of the CODATA Task Group on Fundamental Constants" (PDF). BIPM. p. 7. Archived from the original (PDF) on 2015-10-13.
[BIPM director Martin] Milton responded to a question about what would happen if ... the CIPM or the CGPM voted not to move forward with the redefinition of the SI. He responded that he felt that by that time the decision to move forward should be seen as a foregone conclusion.
- ^ "2022 CODATA Value: Boltzmann constant". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18.
- ^ a b "Resolution 1 of the 26th CGPM (2018)". BIPM. Retrieved 2022-02-21.
- ^ a b Newell, D B; Cabiati, F; Fischer, J; Fujii, K; Karshenboim, S G; Margolis, H S; de Mirandés, E; Mohr, P J; Nez, F; Pachucki, K; Quinn, T J; Taylor, B N; Wang, M; Wood, B M; Zhang, Z; et al. (Committee on Data for Science and Technology (CODATA) Task Group on Fundamental Constants) (2018-01-29). "The CODATA 2017 values of h, e, k, and NA for the revision of the SI". Metrologia. 55 (1): L13–L16. Bibcode:2018Metro..55L..13N. doi:10.1088/1681-7575/aa950a.
- ^ "Updating the definition of the kelvin" (PDF). BIPM. Archived from the original (PDF) on 2008-11-23. Retrieved 2010-02-23.
- ^ Fischer, J; Fellmuth, B; Gaiser, C; Zandt, T; Pitre, L; Sparasci, F; Plimmer, M D; de Podesta, M; Underwood, R; Sutton, G; Machin, G; Gavioso, R M; Madonna Ripa, D; Steur, P P M; Qu, J; Feng, X J; Zhang, J; Moldover, M R; Benz, S P; White, D R; Gianfrani, L; Castrillo, A; Moretti, L; Darquié, B; Moufarej, E; Daussy, C; Briaudeau, S; Kozlova, O; Risegari, L; Segovia, J J; Martín, M C; del Campo, D (2018-04-01). "The Boltzmann project". Metrologia. 55 (2): R1–R20. doi:10.1088/1681-7575/aaa790. PMC 6508687. PMID 31080297.
- ^ "NIST Guide to the SI | Chapter 9: Rules and Style Conventions for Spelling Unit Names", NIST SP 811,
A derived unit is usually singular in English, for example, the value 3 m2·K/W is usually spelled out as 'three square meter kelvin per watt', and the value 3 C·m2/V is usually spelled out as 'three coulomb meter squared per volt'. However, a 'single' unit may be plural; for example, the value 5 kPa is spelled out as 'five kilopascals', although 'five kilopascal' is acceptable. If in such a single-unit case the number is less than one, the unit is always singular when spelled out; for example, 0.5 kPa is spelled out as 'five-tenths kilopascal'.
- ^ "Definition of KELVIN". www.merriam-webster.com. Retrieved 2023-08-21.
- ^ CERN English Language Style Guide (PDF). CERN. 2022. p. 64.
- ^ "Writing with SI (Metric System) Units". NIST. 2010-01-13.
- ^ Brady, James E.; Senese, Fred (2008-01-28). Chemistry, Student Study Guide: The Study of Matter and Its Changes. John Wiley & Sons. p. 15. ISBN 978-0-470-18464-6.
- ^ "22.2". The Unicode Standard, Version 8.0 (PDF). Mountain View, CA, USA: The Unicode Consortium. August 2015. ISBN 978-1-936213-10-8. Archived (PDF) from the original on 2016-12-06. Retrieved 2015-09-06.
Bibliography
- Bureau International des Poids et Mesures (2019). "The International System of Units (SI) Brochure" (PDF). 9th Edition. International Committee for Weights and Measures. Retrieved 2022-04-28.

