Flow cytometry: Difference between revisions
m Reverted edits by 123.252.178.253 (talk) to last version by 153.97.9.52 |
|||
| Line 26: | Line 26: | ||
The data generated by flow-cytometers can be plotted in a single [[dimension]], to produce a [[histogram]], or in two dimensional dot plots or even in three dimensions. The regions on these plots can be sequentially separated, based on fluorescence [[intensity]], by creating a series of subset extractions, termed "gates". Specific gating protocols exist for diagnostic and clinical purposes especially in relation to [[hematology]]. The plots are often made on logarithmic scales. Because different fluorescent dyes' emission spectra overlap [http://pingu.salk.edu/flow/fluo.html], signals at the detectors have to be compensated electronically as well as computationally. Often, data accumulated using the flow cytometer can be re-analysed (using free software e.g. WinMDI [http://facs.scripps.edu/software.html]) elsewhere freeing up the machine for other people to use. |
The data generated by flow-cytometers can be plotted in a single [[dimension]], to produce a [[histogram]], or in two dimensional dot plots or even in three dimensions. The regions on these plots can be sequentially separated, based on fluorescence [[intensity]], by creating a series of subset extractions, termed "gates". Specific gating protocols exist for diagnostic and clinical purposes especially in relation to [[hematology]]. The plots are often made on logarithmic scales. Because different fluorescent dyes' emission spectra overlap [http://pingu.salk.edu/flow/fluo.html], signals at the detectors have to be compensated electronically as well as computationally. Often, data accumulated using the flow cytometer can be re-analysed (using free software e.g. WinMDI [http://facs.scripps.edu/software.html]) elsewhere freeing up the machine for other people to use. |
||
== Fluorescence-Activated Cell Sorting == |
|||
Fluorescence-activated cell sorting is a specialised type of flow cytometry. It provides a method for sorting a heterogeneous mixture of biological [[cells (biology)|cells]] into two or more containers, one cell at a time, based upon the specific [[scattering|light scattering]] and [[fluorescent]] characteristics of each cell. It is a useful scientific instrument as it provides fast, objective and quantitative recording of fluorescent signals from individual cells as well as physical separation of cells of particular interest. The acronym FACS is [[trademark]]ed and owned by [[Becton Dickinson]]<ref> {{cite web|url=http://www.bdbiosciences.com/pdfs/brochures/23-3428-02.pdf |title=FACS MultiSET System |accessdate=2007-02-09 |format=PDF |publisher=Becton Dickinson }}</ref>. While many immunologists use this term frequently for all types of sorting and non-sorting applications, it is not a generic term for flow cytometry. The first cell sorter was invented by Mack Fulwyler in 1965 using the principle of [[Coulter volume]] a relatively difficult technique to use for sorting and one no longer used in modern instruments. The technique was expanded by [[Leonard Herzenberg|Len Herzenberg]] who was responsible for coining the term FACS. Herzenberg won the [[Kyoto Prize]] in 2006 for his work in flow cytometry. |
|||
The cell suspension is entrained in the center of a narrow, rapidly flowing stream of [[liquid]]. The flow is arranged so that there is a large separation between cells relative to their [[diameter]]. A [[oscillation|vibrating]] mechanism causes the stream of cells to break into individual droplets. The system is adjusted so that there is a low probability of more than one cell being in a droplet. Just before the stream breaks into droplets the flow passes through a fluorescence measuring station where the fluorescent character of interest of each cell is measured. An electrical charging ring is placed just at the point where the stream breaks into droplets. A [[electric charge|charge]] is placed on the ring based on the immediately prior fluorescence intensity measurement and the opposite charge is trapped on the droplet as it breaks from the stream. The charged droplets then fall through an [[electrostatic deflection]] system that diverts droplets into containers based upon their charge. In some systems the charge is applied directly to the stream and the droplet breaking off retains charge of the same sign as the stream. The stream is then returned to neutral after the droplet breaks off. |
|||
== Fluorescent labels == |
== Fluorescent labels == |
||
| Line 75: | Line 80: | ||
== Applications == |
== Applications == |
||
The technology has applications in a number of fields, including [[molecular biology]], [[pathology]], [[immunology]], [[plant biology]] and [[marine biology]]. In the field of molecular biology it is especially useful when used with fluorescence tagged antibodies. These specific antibodies bind to [[antigen]]s on the target cells and help to give information on specific characteristics of the cells being studied in the cytometer. It has broad application in [[medicine]] (especially in transplantation, hematology, tumor immunology and chemotherapy, genetics and [[sperm sorting]] for [[sex preselection]]). In marine biology, the auto-fluorescent properties of photosynthetic [[plankton]] can be exploited by flow cytometry in order to characterise abundance and community structure. In protein engineering, flow cytometry is used in conjunction with [[yeast display]] and [[bacterial display]] to identify cell surface-displayed protein variants with desired properties. |
The technology has applications in a number of fields, including [[molecular biology]], [[pathology]], [[immunology]], [[plant biology]] and [[marine biology]]. In the field of molecular biology it is especially useful when used with fluorescence tagged antibodies. These specific antibodies bind to [[antigen]]s on the target cells and help to give information on specific characteristics of the cells being studied in the cytometer. It has broad application in [[medicine]] (especially in transplantation, hematology, tumor immunology and chemotherapy, genetics and [[sperm sorting]] for [[sex preselection]]). In marine biology, the auto-fluorescent properties of photosynthetic [[plankton]] can be exploited by flow cytometry in order to characterise abundance and community structure. In protein engineering, flow cytometry is used in conjunction with [[yeast display]] and [[bacterial display]] to identify cell surface-displayed protein variants with desired properties. |
||
==See also== |
|||
* [[FlowJo]] (software package) |
|||
* [[Fluorescence microscopy]] |
|||
==Bibliography== |
|||
* Flow Cytometry First Principles by Alice Longobardi Givan ISBN 0471382248 |
|||
* Practical Flow Cytometry by Howard M. Shapiro ISBN 0471411256 |
|||
* Flow Cytometry for Biotechnology by Larry A. Sklar ISBN 0195152344 |
|||
* Handbook of Flow Cytometry Methods by J. Paul Robinson, et al. ISBN 0471596345 |
|||
* Current Protocols in Cytometry, Wiley-Liss Pub. {{ISSN|1934-9297}} |
|||
* Flow Cytometry in Clinical Diagnosis, v4, (Carey, McCoy, and Keren, eds), ASCP Press, 2007. ISBN 0891895485 |
|||
* Ormerod, M.G. (ed.) (2000) Flow cytometry - A practical approach. 3rd edition. Oxford University Press, Oxford, UK. ISBN 0199638241 |
|||
* Ormerod, M.G. (1999) Flow Cytometry. 2nd edition. Bios Scientific Publishers, Ltd. Oxford. ISBN 185996107X |
|||
* Flow Cytometry - a Basic Introduction. Michael G. Ormerod, 2008. ISBN 978-0955981203 |
|||
==References== |
|||
{{Reflist}} |
|||
==External links== |
|||
* [http://www.unsolvedmysteries.oregonstate.edu/flow_06 Flow cytometry - How does it work?] |
|||
* [http://probes.invitrogen.com/resources/education/ Tutorials on fluorescence and flow cytometry] |
|||
* {{MeshName|Flow+cytometry}} |
|||
* [http://www.cyto.purdue.edu/flowcyt/educate/pptslide.htm Powerpoint lectures on flow cytometry] |
|||
* [http://sciencepark.mdanderson.org/fcores/flow/files/Operation.html How a flow cytometer operates] |
|||
* [http://www.fluorophores.org Fluorophores.org - The database of fluorescent dyes] |
|||
* [http://pingu.salk.edu/flow/fluo.html Table of fluorochromes] |
|||
* [http://www.bdbiosciences.com/spectra/ Java Fluorescence Spectrum Viewer] |
|||
* [http://www.cshprotocols.org/cgi/content/full/2007/22/pdb.prot4895 Combined Flow Cytometric Measurement of Two Cell-Surface] |
|||
* [http://www.cytometry.org The Clinical Cytometry Society] |
|||
* [http://wiki.clinicalflow.com Clinical Flow Wiki] |
|||
* [http://www.cytologyproject.info High Throughput Flow Cytometry] |
|||
* [http://www.ficcs.org/ FICCS] - the Flow Informatics and Computation Cytometry Society |
|||
* [http://www.amnis.com Amnis] Image Cells in Flow. |
|||
* [http://www.coulterflow.com Coulterflow] Flow Cytometry Tools. |
|||
{{pathology}} |
|||
[[Category:Cell biology]] |
|||
[[Category:Clinical pathology]] |
|||
[[Category:Hematology]] |
|||
[[Category:Medical tests]] |
|||
[[ca:Citometria de flux]] |
|||
[[de:Durchflusszytometrie]] |
|||
[[es:Citometría]] |
|||
[[fr:Cytométrie en flux]] |
|||
[[nl:Flowcytometrie]] |
|||
[[ja:フローサイトメトリー]] |
|||
[[pl:Cytometria przepływowa]] |
|||
[[pt:Citometria de fluxo]] |
|||
[[sv:Flödescytometri]] |
|||
[[uk:Проточна цитометрія]] |
|||
[[zh:流式細胞術]] |
|||
Revision as of 09:08, 10 April 2009
Flow cytometry is a technique for counting, examining, and sorting microscopic particles suspended in a stream of fluid. It allows simultaneous multiparametric analysis of the physical and/or chemical characteristics of single cells flowing through an optical and/or electronic detection apparatus.
History
The first fluorescence-based flow cytometry device (ICP 11) was developed in the year 1968 by Wolfgang Göhde from the University of Münster, Germany (Patent No. DE1815352) and first commercialized in 1968/69 by German developer and manufacturer Partec through Phywe AG in Göttingen. At that time absorption methods were still widely favored by other scientists over fluorescence methods [1]. The original name of the flow cytometry technology was pulse cytophotometry (German: Impulszytophotometrie). Only 10 years later in 1978, at the Conference of the American Engineering Foundation in Pensacola, Florida, the name was changed to flow cytometry, a term which quickly became popular. Subsequently introduced flow cytometry instruments have been the Cytofluorograph (1971) from Bio/Physics Systems Inc. (later: Ortho Diagnostics), the PAS 8000 (1973) from Partec, the first FACS instrument from Becton Dickinson (1974), the ICP 22 (1975) from Partec/Phywe and the Epics from Coulter (1977/78).
Principle of flow cytometry
A beam of light (usually laser light) of a single wavelength is directed onto a hydro-dynamically focused stream of fluid. A number of detectors are aimed at the point where the stream passes through the light beam; one in line with the light beam (Forward Scatter or FSC) and several perpendicular to it (Side Scatter (SSC) and one or more fluorescent detectors). Each suspended particle from 0.2 to 150 micrometers passing through the beam scatters the light in some way, and fluorescent chemicals found in the particle or attached to the particle may be excited into emitting light at a higher wavelength than the light source. This combination of scattered and fluorescent light is picked up by the detectors, and by analysing fluctuations in brightness at each detector (one for each fluorescent emission peak) it is then possible to derive various types of information about the physical and chemical structure of each individual particle. FSC correlates with the cell volume and SSC depends on the inner complexity of the particle (i.e. shape of the nucleus, the amount and type of cytoplasmic granules or the membrane roughness). Some flow cytometers on the market have eliminated the need for fluorescence and use only light scatter for measurement. Other flow cytometers form images of each cell's fluorescence, scattered light, and transmitted light.
Flow cytometers
Modern flow cytometers are able to analyse several thousand particles every second, in "real time", and can actively separate and isolate particles having specified properties. A flow cytometer is similar to a microscope, except that instead of producing an image of the cell, flow cytometry offers "high-throughput" (for a large number of cells) automated quantification of set parameters. To analyze solid tissues single-cell suspension must first be prepared.
A flow cytometer has 5 main components:
- a flow cell - liquid stream (sheath fluid) carries and aligns the cells so that they pass single file through the light beam for sensing.
- an optical system - commonly used are lamps (mercury, xenon); high power water-cooled lasers (argon, krypton, dye laser); low power air-cooled lasers (argon (488nm), red-HeNe (633nm), green-HeNe, HeCd (UV)); diode lasers (blue, green, red, violet) resulting in light signals.
- a detector and Analogue-to-Digital Conversion (ADC) system - generating FSC and SSC as well as fluorescence signals from light into electrical signals that can be processed by a computer.
- an amplification system - linear or logarithmic.
- a computer for analysis of the signals.
Early flow cytometers were generally experimental devices, but recent technological advances have created a considerable market for the instrumentation, as well as the reagents used in analysis, such as fluorescently-labeled antibodies and analysis software.
Modern instruments usually have multiple lasers and fluorescence detectors (the current record for a commercial instrument is 4 lasers and 18 fluorescence detectors). Increasing the number of lasers and detectors allows for multiple antibody labelling, and can more precisely identify a target population by their phenotype. Certain instruments can even take digital images of individual cells, allowing for the analysis of fluorescent signal location within or on the surface of cells.
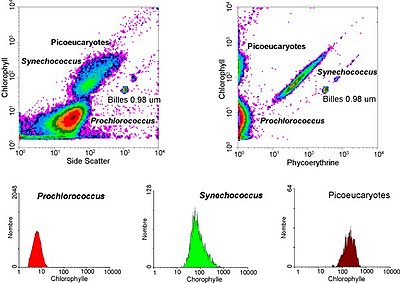
The data generated by flow-cytometers can be plotted in a single dimension, to produce a histogram, or in two dimensional dot plots or even in three dimensions. The regions on these plots can be sequentially separated, based on fluorescence intensity, by creating a series of subset extractions, termed "gates". Specific gating protocols exist for diagnostic and clinical purposes especially in relation to hematology. The plots are often made on logarithmic scales. Because different fluorescent dyes' emission spectra overlap [1], signals at the detectors have to be compensated electronically as well as computationally. Often, data accumulated using the flow cytometer can be re-analysed (using free software e.g. WinMDI [2]) elsewhere freeing up the machine for other people to use.
Fluorescence-Activated Cell Sorting
Fluorescence-activated cell sorting is a specialised type of flow cytometry. It provides a method for sorting a heterogeneous mixture of biological cells into two or more containers, one cell at a time, based upon the specific light scattering and fluorescent characteristics of each cell. It is a useful scientific instrument as it provides fast, objective and quantitative recording of fluorescent signals from individual cells as well as physical separation of cells of particular interest. The acronym FACS is trademarked and owned by Becton Dickinson[2]. While many immunologists use this term frequently for all types of sorting and non-sorting applications, it is not a generic term for flow cytometry. The first cell sorter was invented by Mack Fulwyler in 1965 using the principle of Coulter volume a relatively difficult technique to use for sorting and one no longer used in modern instruments. The technique was expanded by Len Herzenberg who was responsible for coining the term FACS. Herzenberg won the Kyoto Prize in 2006 for his work in flow cytometry.
The cell suspension is entrained in the center of a narrow, rapidly flowing stream of liquid. The flow is arranged so that there is a large separation between cells relative to their diameter. A vibrating mechanism causes the stream of cells to break into individual droplets. The system is adjusted so that there is a low probability of more than one cell being in a droplet. Just before the stream breaks into droplets the flow passes through a fluorescence measuring station where the fluorescent character of interest of each cell is measured. An electrical charging ring is placed just at the point where the stream breaks into droplets. A charge is placed on the ring based on the immediately prior fluorescence intensity measurement and the opposite charge is trapped on the droplet as it breaks from the stream. The charged droplets then fall through an electrostatic deflection system that diverts droplets into containers based upon their charge. In some systems the charge is applied directly to the stream and the droplet breaking off retains charge of the same sign as the stream. The stream is then returned to neutral after the droplet breaks off.
Fluorescent labels
The fluorescence labels that can be used, will depend on the lamp or laser used to excite the fluorochromes and on the detectors available:[3]
- Blue Argon Laser (488 nm)
This is an air cooled laser and therefore cheaper to set up and run. It is the most commonly available laser on single laser machines.
- Green (usually labelled FL1): FITC, Alexa Fluor 488, GFP, CFSE, CFDA-SE, DyLight 488
- Orange (usually FL2): PE, PI
- Red channel (usually FL3): PerCP, PE-Alexa Fluor 700, PE-Cy5 (TRI-COLOR), PE-Cy5.5.
- Infra-red (usually FL4; not provided by all FACS machines as standard): PE-Alexa Fluor 750, PE-Cy7
Red diode laser (635 nm)
- APC
- APC-Cy7
- Alexa Fluor 700
- Cy5
- Draq-5
Violet laser (405 nm)
- Pacific Orange
- Amine Aqua
- Pacific Blue
- DAPI
- Alexa Fluor 405
Measurable parameters
This list is very long and constantly expanding.
- volume and morphological complexity of cells
- cell pigments such as chlorophyll or phycoerythrin
- DNA (cell cycle analysis, cell kinetics, proliferation etc.)
- RNA
- chromosome analysis and sorting (library construction, chromosome paint)
- protein expression and localization
- Protein modifications, phospho-proteins
- transgenic products in vivo, particularly the Green fluorescent protein or related fluorescent * cell surface antigens (Cluster of differentiation (CD) markers)
- intracellular antigens (various cytokines, secondary mediators etc.)
- nuclear antigens
- enzymatic activity
- pH, intracellular ionized calcium, magnesium, membrane potential
- membrane fluidity
- apoptosis (quantification, measurement of DNA degradation, mitochondrial membrane potential, permeability changes, caspase activity)
- cell viability
- monitoring electropermeabilization of cells
- oxidative burst
- characterising multidrug resistance (MDR) in cancer cells
- glutathione
- various combinations (DNA/surface antigens etc.)
Applications
The technology has applications in a number of fields, including molecular biology, pathology, immunology, plant biology and marine biology. In the field of molecular biology it is especially useful when used with fluorescence tagged antibodies. These specific antibodies bind to antigens on the target cells and help to give information on specific characteristics of the cells being studied in the cytometer. It has broad application in medicine (especially in transplantation, hematology, tumor immunology and chemotherapy, genetics and sperm sorting for sex preselection). In marine biology, the auto-fluorescent properties of photosynthetic plankton can be exploited by flow cytometry in order to characterise abundance and community structure. In protein engineering, flow cytometry is used in conjunction with yeast display and bacterial display to identify cell surface-displayed protein variants with desired properties.
See also
- FlowJo (software package)
- Fluorescence microscopy
Bibliography
- Flow Cytometry First Principles by Alice Longobardi Givan ISBN 0471382248
- Practical Flow Cytometry by Howard M. Shapiro ISBN 0471411256
- Flow Cytometry for Biotechnology by Larry A. Sklar ISBN 0195152344
- Handbook of Flow Cytometry Methods by J. Paul Robinson, et al. ISBN 0471596345
- Current Protocols in Cytometry, Wiley-Liss Pub. ISSN 1934-9297
- Flow Cytometry in Clinical Diagnosis, v4, (Carey, McCoy, and Keren, eds), ASCP Press, 2007. ISBN 0891895485
- Ormerod, M.G. (ed.) (2000) Flow cytometry - A practical approach. 3rd edition. Oxford University Press, Oxford, UK. ISBN 0199638241
- Ormerod, M.G. (1999) Flow Cytometry. 2nd edition. Bios Scientific Publishers, Ltd. Oxford. ISBN 185996107X
- Flow Cytometry - a Basic Introduction. Michael G. Ormerod, 2008. ISBN 978-0955981203
References
- ^ Kamentsky, Proceedings of the Conference „Cytology Automation" in Edinburgh, 1970
- ^ "FACS MultiSET System" (PDF). Becton Dickinson. Retrieved 2007-02-09.
- ^ Loken MR (1990). "Immunofluorescence Techniques in Flow Cytometry and Sorting" (2nd ed.). Wiley: 341–53.
{{cite journal}}: Cite journal requires|journal=(help)
External links
- Flow cytometry - How does it work?
- Tutorials on fluorescence and flow cytometry
- Flow+cytometry at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Powerpoint lectures on flow cytometry
- How a flow cytometer operates
- Fluorophores.org - The database of fluorescent dyes
- Table of fluorochromes
- Java Fluorescence Spectrum Viewer
- Combined Flow Cytometric Measurement of Two Cell-Surface
- The Clinical Cytometry Society
- Clinical Flow Wiki
- High Throughput Flow Cytometry
- FICCS - the Flow Informatics and Computation Cytometry Society
- Amnis Image Cells in Flow.
- Coulterflow Flow Cytometry Tools.
