Josiah Willard Gibbs: Difference between revisions
m Added the {{Authority control}} template with VIAF number 122317710: http://viaf.org/viaf/122317710 . Please report any errors. |
→Influence: Clarifying that "convex analysis" is math, not thermodynamics |
||
| (139 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
| ⚫ | |||
{{about||Josiah Willard Gibbs, Sr.|Josiah Willard Gibbs, Sr.|the United States Navy ship|USNS Josiah Willard Gibbs (T-AGOR-1)}} |
{{about||Josiah Willard Gibbs, Sr.|Josiah Willard Gibbs, Sr.|the United States Navy ship|USNS Josiah Willard Gibbs (T-AGOR-1)}} |
||
{{Infobox scientist |
{{Infobox scientist |
||
|name = J. Willard Gibbs |
|name = J. Willard Gibbs |
||
| Line 32: | Line 30: | ||
}} |
}} |
||
'''Josiah Willard Gibbs''' (February 11, 1839 – April 28, 1903) was an American scientist who made important theoretical contributions to physics, chemistry, and mathematics. His work on the applications of [[thermodynamics]] was instrumental in transforming [[physical chemistry]] into a rigorous deductive science. Together with [[James Clerk Maxwell]] and [[Ludwig Boltzmann]], he created [[statistical mechanics]] (a term that he coined), explaining the laws of thermodynamics |
'''Josiah Willard Gibbs''' (February 11, 1839 – April 28, 1903) was an American scientist who made important theoretical contributions to physics, chemistry, and mathematics. His work on the applications of [[thermodynamics]] was instrumental in transforming [[physical chemistry]] into a rigorous deductive science. Together with [[James Clerk Maxwell]] and [[Ludwig Boltzmann]], he created [[statistical mechanics]] (a term that he coined), explaining the laws of thermodynamics as consequences of the statistical properties of large [[Statistical ensemble (mathematical physics)|ensembles]] of particles. Gibbs also worked on the application of [[Maxwell's equations]] to problems in [[physical optics]]. As a mathematician, he invented modern [[vector calculus]] (independently of the British scientist [[Oliver Heaviside]], who carried out similar work during the same period). |
||
In 1863, [[Yale University]] awarded Gibbs the first American [[Doctor of Philosophy|doctorate]] in [[engineering]]. After a three-year sojourn in Europe, Gibbs spent the rest of his career at Yale, where he was professor of mathematical physics. Working in relative isolation, he became the earliest theoretical scientist in the United States to earn an international reputation and was praised by [[Albert Einstein]] as "the greatest mind in American history |
In 1863, [[Yale University]] awarded Gibbs the first American [[Doctor of Philosophy|doctorate]] in [[engineering]]. After a three-year sojourn in Europe, Gibbs spent the rest of his career at Yale, where he was professor of mathematical physics from 1871 until his death. Working in relative isolation, he became the earliest theoretical scientist in the United States to earn an international reputation and was praised by [[Albert Einstein]] as "the greatest mind in American history".<ref name="APS">{{cite web |url=http://www.aps.org/programs/outreach/history/historicsites/gibbs.cfm |title=J. Willard Gibbs |author= |date= |work=Physics History |publisher=American Physical Society |accessdate=16 Jun. 2012}}</ref> In 1901 Gibbs received what was then considered the highest honor awarded by the international scientific community, the [[Copley Medal]] of the [[Royal Society]] of London,<ref name="APS" /> "for his contributions to mathematical physics".<ref name="Copley">{{cite web |url=http://royalsociety.org/awards/copley-medal/ |title=Copley Medal |author= |date= |work=Premier Awards |publisher=Royal Society |accessdate=16 Jun. 2012}}</ref> |
||
| ⚫ | Commentators and biographers have remarked on the contrast between Gibbs's quiet, solitary life in turn of the century [[New England]] and the great international impact of his ideas. Though his work was almost entirely theoretical, the practical value of Gibbs's contributions became evident with the development of industrial chemistry during the first half of the 20th century. According to [[Robert Andrews Millikan|Robert A. Millikan]], in pure science Gibbs "did for statistical mechanics and for thermodynamics what [[Pierre-Simon Laplace|Laplace]] did for celestial mechanics and Maxwell did for electrodynamics, namely, made his field a well-nigh finished theoretical structure."<ref name="Millikan">{{cite journal | last=Millikan |first=Robert A. |authorlink=Robert Andrews Millikan |year=1938 |title=Biographical Memoir of Albert Abraham Michelson, 1852–1931 |journal=Biographical Memoirs of the National Academy of Sciences of the United States of America |volume=19 |issue=4 |pages=121–146| url=http://books.nap.edu/html/biomems/amichelson.pdf}}</ref> |
||
== Biography == |
== Biography == |
||
=== Family background === |
=== Family background === |
||
Gibbs was the fourth of |
Gibbs was the fourth of five children and the only son of [[Josiah Willard Gibbs, Sr.|Josiah Willard Gibbs]] and his wife Mary Anna, ''née'' Van Cleve. The father was a linguist and theologian who served as professor of sacred literature at [[Yale Divinity School]] from 1824 until his death in 1861. He was an active [[abolitionism|abolitionist]] and is now chiefly remembered for finding an interpreter for the African passengers of the ship ''[[La Amistad|Amistad]]'', allowing them to testify during [[United States v. The Amistad|the trial]] that followed their rebellion against being sold as slaves.<ref>{{cite web |url=http://law2.umkc.edu/faculty/projects/ftrials/amistad/AMI_BGIB.HTM |title=Biography of Prof. Josiah Gibbs |last = Linder | first = Douglas |date= |work=Famous American Trials: Amistad Trial |publisher=University of Missouri-Kansas City School of Law |accessdate=16 Jun. 2012}}</ref> |
||
The younger Willard Gibbs belonged to a long line of American academics and clergymen that stretched back to the 17th century. On his father's side, he was descended from [[Samuel Willard]], who served as acting [[President of Harvard University]] from 1701 to 1707. On his mother's side, one of his ancestors was the Rev. [[Jonathan Dickinson (New Jersey)|Jonathan Dickinson]], who was the first president of the College of New Jersey (later [[Princeton University]]). His given name, which he shared with his father and several other members of his extended family, derived from his ancestor Josiah Willard, who had been Secretary of the [[Province of Massachusetts Bay]] in the 18th century.<ref name="Bumstead"> |
The younger Willard Gibbs belonged to a long line of American academics and clergymen that stretched back to the 17th century. On his father's side, he was descended from [[Samuel Willard]], who served as acting [[President of Harvard University]] from 1701 to 1707. On his mother's side, one of his ancestors was the Rev. [[Jonathan Dickinson (New Jersey)|Jonathan Dickinson]], who was the first president of the College of New Jersey (later [[Princeton University]]). His given name, which he shared with his father and several other members of his extended family, derived from his ancestor Josiah Willard, who had been Secretary of the [[Province of Massachusetts Bay]] in the 18th century.<ref name="Bumstead">Bumstead 1928</ref> |
||
===Early years=== |
===Early years=== |
||
[[File:JWGibbs-student.jpg|thumb|left|JWGibbs-student|Willard Gibbs as a student, circa 1855]] |
[[File:JWGibbs-student.jpg|thumb|left|JWGibbs-student|Willard Gibbs as a student, circa 1855]] |
||
Gibbs was educated at the [[Hopkins School]] and entered [[Yale College]] in 1854, |
Gibbs was educated at the [[Hopkins School]] and entered [[Yale College]] in 1854, aged 15. He graduated in 1858 near the top of his class, and was awarded prizes for excellence in [[mathematics]] and [[Latin]].<ref name="MacTutor">{{cite web |url=http://www-groups.dcs.st-and.ac.uk/history/Biographies/Gibbs.html |title=Josiah Willard Gibbs | last1 = O'Connor | first1 = John J. | last2 = Robertson | first2 = Edmund F. | year = 1997 | work=The MacTutor History of Mathematics archive |publisher=University of St Andrews, Scotland. School of Mathematics and Statistics |accessdate=16 Jun. 2012}}</ref> He remained at Yale as a graduate student at the [[Sheffield Scientific School]]. At the age of 19, soon after his graduation from college, Gibbs was inducted into the Connecticut Academy of Arts and Sciences, a scholarly institution composed primarily of members of the Yale faculty.<ref name="Rukeyser-CTAcademy">Rukeyser 1988, p. 104</ref> |
||
After the death of his father in 1861, Gibbs inherited enough money to make him financially independent.<ref name="Rukeyser-inheritance">Rukeyser 1998, pp. |
Relatively few documents from the period survive and it is impossible to reconstruct the details of Gibbs's early career with precision.<ref>Rukeyser 1988, p. 430</ref><ref name="Wheeler-college">Wheeler 1998, pp. 23–4</ref> In the opinion of biographers, Gibbs's principal mentor and champion, in both the University and the Connecticut Academy, was probably the astronomer and mathematician [[Hubert Anson Newton]], a leading authority on [[Meteoroid|meteors]], who remained Gibbs's lifelong friend and confidant.<ref name="Rukeyser-CTAcademy" /><ref name="Wheeler-college" /> After the death of his father in 1861, Gibbs inherited enough money to make him financially independent.<ref name="Rukeyser-inheritance">Rukeyser 1998, pp. 120, 142</ref> He suffered from recurrent pulmonary trouble as a young man and his doctors were concerned that he might be susceptible to [[tuberculosis]], which had killed his mother. These problems and a defect in his eyesight probably explain why he did not volunteer to fight in the [[American Civil War|Civil War]] of 1861–65.<ref name="Wheeler-war">Wheeler 1998, p. 30</ref> He was not [[Conscription in the United States#Civil War|conscripted]] and he remained at Yale for the duration of the war.<ref name="Rukeyser-war">Rukeyser 1998, p. 134</ref> |
||
In 1863, Gibbs received the first [[Doctor of Philosophy|Ph.D. degree]] in [[engineering]] granted in the US, for a thesis entitled "On the Form of the Teeth of Wheels in Spur Gearing |
In 1863, Gibbs received the first [[Doctor of Philosophy|Ph.D. degree]] in [[engineering]] granted in the US, for a thesis entitled "On the Form of the Teeth of Wheels in Spur Gearing", in which he used geometrical techniques to investigate the optimum design for [[gear]]s.<ref name="Wheeler-PhD">Wheeler 1998, p. 32</ref> This was also the fifth Ph.D. granted in the US in any subject.<ref name="Wheeler-PhD"/> After graduation, Gibbs was appointed as tutor at the College for a term of three years. During the first two years he taught Latin and during the third Natural Philosophy (i.e., physics).<ref name="Bumstead" /> In 1866 he patented a design for a [[railway brake]]<ref>US Patent No. 53,971, "Car Brake", Apr. 17, 1866. See ''The Early Work of Willard Gibbs in Applied Mechanics'', (New York: Henry Schuman, 1947), pp. 51–62.</ref> and read a paper before the Connecticut Academy, entitled "The Proper Magnitude of the Units of Length", in which he proposed a scheme for rationalizing the system of units of measurement used in mechanics.<ref name="Units">Wheeler 1998, appendix II</ref> |
||
[[Image:Thermodynamicist Willard Gibbs.jpg|thumb|upright|140px|Gibbs during his time as a tutor at Yale<ref>Wheeler 1998, p. 44</ref>]] |
[[Image:Thermodynamicist Willard Gibbs.jpg|thumb|upright|140px|Gibbs during his time as a tutor at Yale<ref>Wheeler 1998, p. 44</ref>]] |
||
After his term as tutor ended, Gibbs travelled to Europe with his sisters, spending the winter of 1866–67 in Paris, where he attended lectures at the [[University of Paris|Sorbonne]] and the [[Collège de France]]. From there he went to [[Berlin]], where he attended the lectures of [[Heinrich Gustav |
After his term as tutor ended, Gibbs travelled to Europe with his sisters, spending the winter of 1866–67 in Paris, where he attended lectures at the [[University of Paris|Sorbonne]] and the [[Collège de France]]. From there he went to [[Berlin]], where he attended the lectures of [[Heinrich Gustav Magnus]], and to [[Heidelberg]], where he was exposed to the scientific work of [[Gustav Kirchhoff]] and [[Hermann von Helmholtz]]. At the time, German academics were the leading authorities in [[chemistry]], [[thermodynamics]], and natural science in general.<ref>Wheeler 1998, ch. III</ref> |
||
Gibbs returned to Yale in June 1869 and briefly taught French to engineering students.<ref name="Klein-proceedings"> |
Gibbs returned to Yale in June 1869 and briefly taught French to engineering students.<ref name="Klein-proceedings">{{cite book | chapter = The Physics of J. Willard Gibbs in His Time | last = Klein | first = Martin J. | title = Proceedings of the Gibbs Symposium | year = 1990 |pages = 1–22}}</ref> It was probably also around this time that he worked on a new design for a steam-engine [[Governor (device)|governor]], his last significant investigation in mechanical engineering.<ref name="Mayr">{{Cite jstor|531164}}</ref><ref>Wheeler 1998, pp. 54–5</ref> In 1871 he was appointed Professor of Mathematical Physics at Yale, the first such professorship in the United States. Gibbs, who had independent means and had yet to publish anything, was assigned to teach graduate students exclusively and was hired without salary.<ref name="Rukeyser-professor">Rukeyser 1988, pp. 181–2</ref> Unsalaried teaching positions were common in German universities, on which the system of graduate scientific instruction at Yale was then being modeled.<ref name="Wheeler-professor">Wheeler 1998, pp. 57–9</ref> |
||
===Middle years=== |
===Middle years=== |
||
[[File:Maxwell's letters plate IV.jpg|thumb|250px|left| |
[[File:Maxwell's letters plate IV.jpg|thumb|250px|left|Maxwell's sketch of the lines of constant temperature and pressure, made in preparation for his construction of a solid model based on Gibbs's definition of a thermodynamic surface for water (see [[Maxwell's thermodynamic surface]])]] |
||
Gibbs |
Gibbs published his first work in 1873, at the unusually advanced age of 34.<ref name="MacTutor" /> His paper on the geometric representation of thermodynamic quantities appeared in the ''Transactions of the Connecticut Academy''. This journal had few readers capable of understanding Gibbs's work, but he shared reprints with his correspondents in Europe and received a very favorable response from [[James Clerk Maxwell]], at the [[University of Cambridge]]. Maxwell even made three plaster casts illustrating Gibbs's construct with his own hands and mailed one to Gibbs. That model is on permanent display at the Yale physics department.<ref>{{cite web |url=http://www.sv.vt.edu/classes/ESM4714/methods/Gibbs.html |title=Thermodynamic Case Study: Gibbs' Thermodynamic Graphical Method |last = Kriz | first = Ronald D. |year=2007 |work= |publisher=Virginia Tech, Dept. of Engineering Science and Mechanics |accessdate=16 Jun. 2012}}</ref> |
||
Maxwell included a new chapter on Gibbs's work in the next edition of his ''Theory of Heat'', published in 1875. He explained the usefulness of Gibbs's graphical methods in a lecture to the [[Chemical Society]] of London and even referred to it in the article on "Diagrams" that he wrote for the ''[[Encyclopædia Britannica]]''.<ref>Rukeyser 1988, p. 201</ref> Maxwell's early death in 1879, at the age of 48, precluded further collaboration between him and Gibbs. The joke later circulated in New Haven that "only one man lived who could understand Gibbs's papers. That was Maxwell, and now he is dead."<ref>Rukeyser 1988, p. 251</ref> |
|||
| ⚫ | |||
Gibbs extended his thermodynamic analysis to multi-phase chemical systems (i.e., to systems composed of more than one kind of matter) and considered a variety of concrete applications. He described that research in a long monograph titled "[[On the Equilibrium of Heterogeneous Substances]]", published in two parts by the Connecticut Academy between 1874 and 1878. This work begins with a quotation taken from the work of [[Rudolf Clausius]] that expresses what would later be called the first and second [[laws of thermodynamics]]: "The [[energy]] of the world is constant. The [[entropy]] of the world tends towards a maximum."<ref>Quoted in Rukeyser 1988, p. 233</ref> |
|||
| ⚫ | {{quote|It is universally recognised that its publication was an event of the first importance in the history of |
||
| ⚫ | Gibbs's monograph is now deemed to be one of the greatest scientific achievements of the 19th century and one of the foundations of modern physical chemistry.<ref name="Bumstead" /> In that work, Gibbs rigorously and ingeniously used his graphic thermodynamic techniques to interpret physico-chemical phenomena, explaining and interrelating what had previously been a mass of isolated facts and observations.<ref name="Wheeler-thermodynamics">Wheeler 1998, ch. V</ref> |
||
| ⚫ | {{quote|It is universally recognised that its publication was an event of the first importance in the history of chemistry... Nevertheless it was a number of years before its value was generally known, this delay was due largely to the fact that its mathematical form and rigorous deductive processes make it difficult reading for anyone, and especially so for students of experimental chemistry whom it most concerns. | J. J. O'Connor and E. F. Robertson<ref name="MacTutor" />}} |
||
Gibbs continued to work without pay until 1880, when the new [[Johns Hopkins University]] in [[Baltimore, Maryland]] offered him a position paying $3,000 per year. In response, Yale offered him a salary of $2,000, which he was content to accept.<ref>Wheeler 1998, p. 91</ref> |
Gibbs continued to work without pay until 1880, when the new [[Johns Hopkins University]] in [[Baltimore, Maryland]] offered him a position paying $3,000 per year. In response, Yale offered him a salary of $2,000, which he was content to accept.<ref>Wheeler 1998, p. 91</ref> |
||
| Line 71: | Line 76: | ||
===Later years=== |
===Later years=== |
||
From 1880 to 1884, Gibbs worked on developing the [[exterior algebra]] of [[Hermann Grassmann]] into a [[vector calculus]] well-suited to the needs of physicists. |
From 1880 to 1884, Gibbs worked on developing the [[exterior algebra]] of [[Hermann Grassmann]] into a [[vector calculus]] well-suited to the needs of physicists. With this object in mind, Gibbs distinguished between the [[Dot product|dot]] and [[cross product]]s of two vectors and introduced the concept of [[dyadics]]. Similar work was carried out independently, and at around the same time, by the British mathematical physicist and engineer [[Oliver Heaviside]]. Gibbs sought to convince other physicists of the convenience of the vectorial approach over the [[quaternion]]ic calculus of [[William Rowan Hamilton]], which was then widely used by British scientists. This led him, in the early 1890s, to a controversy with [[Peter Guthrie Tait]] and others in the pages of ''[[Nature (journal)|Nature]]''.<ref name="Bumstead" /> |
||
[[File:Sine integral.svg|thumb|The [[sine integral]], which gives the overshoot associated with the [[Gibbs phenomenon]] for the Fourier series of a [[Heaviside step function|step function]] on the real line.]] |
[[File:Sine integral.svg|thumb|The [[sine integral]], which gives the overshoot associated with the [[Gibbs phenomenon]] for the Fourier series of a [[Heaviside step function|step function]] on the real line.]] |
||
| Line 77: | Line 82: | ||
Gibbs's lecture notes on vector calculus were privately printed in 1881 and 1884 for the use of his students, and were later adapted by [[Edwin Bidwell Wilson]] into a textbook, ''[[Vector Analysis]]'', published in 1901.<ref name="Bumstead" /> That book helped to popularize the notation that is widely used today in [[Classical electromagnetism|electrodynamics]] and [[fluid mechanics]] (see [[Del|del operator]]). In other mathematical work, he re-discovered the "[[Gibbs phenomenon]]" in the theory of [[Fourier series]] (which, unbeknownst to him and to later scholars, had been described fifty years before by an obscure English mathematician, [[Henry Wilbraham]]).<ref>{{cite doi|10.1007/BF00330404}}</ref> |
Gibbs's lecture notes on vector calculus were privately printed in 1881 and 1884 for the use of his students, and were later adapted by [[Edwin Bidwell Wilson]] into a textbook, ''[[Vector Analysis]]'', published in 1901.<ref name="Bumstead" /> That book helped to popularize the notation that is widely used today in [[Classical electromagnetism|electrodynamics]] and [[fluid mechanics]] (see [[Del|del operator]]). In other mathematical work, he re-discovered the "[[Gibbs phenomenon]]" in the theory of [[Fourier series]] (which, unbeknownst to him and to later scholars, had been described fifty years before by an obscure English mathematician, [[Henry Wilbraham]]).<ref>{{cite doi|10.1007/BF00330404}}</ref> |
||
From 1882 to 1889, Gibbs wrote five papers on [[physical optics]], in which he investigated [[birefringence]] and other optical phenomena and defended Maxwell's electromagnetic theory of light against the mechanical theories of [[William Thomson, 1st Baron Kelvin|Kelvin]] and others.<ref name="Bumstead" /> In his work on optics just as much as in his work on thermodynamics, Gibbs deliberately avoided speculating about the microscopic structure of matter, which proved a wise course in view of the revolutionary developments in [[quantum mechanics]] that began around the time of his death.<ref name="Optics">Wheeler 1998, ch. VIII</ref> |
From 1882 to 1889, Gibbs wrote five papers on [[physical optics]], in which he investigated [[birefringence]] and other optical phenomena and defended Maxwell's electromagnetic theory of light against the mechanical theories of [[William Thomson, 1st Baron Kelvin|Lord Kelvin]] and others.<ref name="Bumstead" /> In his work on optics just as much as in his work on thermodynamics, Gibbs deliberately avoided speculating about the microscopic structure of matter, which proved a wise course in view of the revolutionary developments in [[quantum mechanics]] that began around the time of his death.<ref name="Optics">Wheeler 1998, ch. VIII</ref> |
||
Gibbs coined the term |
Gibbs coined the term ''statistical mechanics'' and introduced key concepts in the corresponding mathematical description of physical systems, including the notions of [[chemical potential]] (1876), [[Statistical ensemble (mathematical physics)|statistical ensemble]] (1878), and [[phase space]] (1902).<ref name="Klein-bio">Klein 2008</ref><ref name="Wheeler-statistical">Wheeler 1998, ch. X</ref> Gibbs's derivation of the phenomenological laws of thermodynamics from the statistical properties of systems with many particles was presented in his highly-influential textbook ''Elementary Principles in Statistical Mechanics'', published in 1902, a year before his death.<ref name="Klein-bio" /> |
||
Gibbs |
Gibbs's retiring personality and intense focus on his work limited his accessibility to students. His principal protégé was Edwin Bidwell Wilson, who nonetheless explained that "except in the classroom I saw very little of Gibbs. He had a way, toward the end of the afternoon, of taking a stroll about the streets between his study in the old Sloane Laboratory and his home—a little exercise between work and dinner—and one might occasionally come across him at that time."<ref name="Wilson-reminiscences ">Wilson 1931</ref> Gibbs did supervise the doctoral thesis on mathematical economics written by [[Irving Fisher]] in 1891.<ref name="Fisher">{{cite journal | last=Fisher |first= Irving |authorlink=Irving Fisher |year=1930 |title=The application of mathematics to the social sciences |journal=Bulletin of the American Mathematical Society |volume=36 |issue=4 |pages=225–243| url=http://projecteuclid.org/DPubS/Repository/1.0/Disseminate?view=body&id=pdf_1&handle=euclid.bams/1183493954 |format=PDF| doi=10.1090/S0002-9904-1930-04919-8}}</ref> After Gibbs's death, Fisher financed the publication of his ''Collected Works''.<ref name="Celebrating-Fisher">{{cite book | last = Fisher | first = George W. | chapter = Foreword | title = Celebrating Irving Fisher: The Legacy of a Great Economist | publisher = Wiley-Blackwell | year = 2005 | url=http://cowles.econ.yale.edu/books/gean/fisher.htm}}</ref> Another distinguished student was [[Lee De Forest]], later a pioneer of radio technology.<ref name="DeForest">{{cite news |title=The man who invented radio | last = Schiff | first = Judith Ann |url=http://www.yalealumnimagazine.com/issues/2008_11/old_yale.html |newspaper= Yale Alumni Magazine |date= Nov./Dec. 2008 |accessdate=24 Jun. 2012}}</ref> |
||
Gibbs died in New Haven, aged 64, the victim of an acute intestinal obstruction.<ref name="Wilson-reminiscences " /> He is buried in [[Grove Street Cemetery]].<ref name="grave">{{cite web |url=http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=18663052 |title=Josiah Willard Gibbs |author= |date= |work= |publisher=Find A Grave |accessdate=19 |
Gibbs died in New Haven, aged 64, the victim of an acute intestinal obstruction.<ref name="Wilson-reminiscences " /> He is buried in [[Grove Street Cemetery]].<ref name="grave">{{cite web |url=http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=18663052 |title=Josiah Willard Gibbs |author= |date= |work= |publisher=Find A Grave |accessdate=19 Jun. 2012}}</ref> |
||
===Personal life and character=== |
===Personal life and character=== |
||
[[File:JWGibbs.jpg|thumb|200px|Portrait of Prof. J. Willard Gibbs, taken around 1895. |
[[File:JWGibbs.jpg|thumb|200px|Portrait of Prof. J. Willard Gibbs, taken around 1895. According to his student Lynde Wheeler, of the existing portraits this is the most faithful to Gibbs's kindly habitual expression.<ref name="Wheeler-portrait">Wheeler 1998, pp. 179–180</ref>]] |
||
Gibbs never married, living all his life in his childhood home with his sister Julia and her husband Addison Van Name, who was the Yale librarian. Except for his customary summer vacations in the [[Adirondack Mountains|Adirondack]]s (at [[Keene Valley, New York]]) and later at the [[White Mountains (New Hampshire)|White Mountains]] (in [[Intervale, New Hampshire]]),<ref name="Seeger-gentleman">Seeger 1974, pp. |
Gibbs never married, living all his life in his childhood home with his sister Julia and her husband Addison Van Name, who was the Yale librarian. Except for his customary summer vacations in the [[Adirondack Mountains|Adirondack]]s (at [[Keene Valley, New York]]) and later at the [[White Mountains (New Hampshire)|White Mountains]] (in [[Intervale, New Hampshire]]),<ref name="Seeger-gentleman">Seeger 1974, pp. 15–6</ref> his sojourn in Europe in 1866–69 was almost the only time that Gibbs spent outside New Haven.<ref name="Bumstead" /> |
||
Gibbs joined Yale's College Church (a [[Congregational church]]) at the end of his freshman year<ref name="Seeger-gentleman" /><ref name="obituary-church">{{cite book | title = Obituary Record of Graduates of Yale University, |
Gibbs joined Yale's College Church (a [[Congregational church]]) at the end of his freshman year<ref name="Seeger-gentleman" /><ref name="obituary-church">{{cite book | title = Obituary Record of Graduates of Yale University, 1901–1910 | publisher = Tuttle, Morehouse & Taylor | year = 1910 | location = New Haven | url = http://books.google.com/books?id=rVkdAQAAIAAJ&pg=PA238 | page = 238}}</ref> and remained a regular attendant for the rest of his life.<ref name="Wheeler-views">Wheeler, 1998, p. 16</ref> He generally voted for the [[Republican Party (United States)|Republican]] candidate in presidential elections, but he supported [[Grover Cleveland]], a conservative [[Democratic Party (United States)|Democrat]].<ref name="Samuelson-politics">{{cite book | last = Samuelson | first = Paul A. | authorlink=Paul Samuelson | chapter = Gibbs in Economics | title = Proceedings of the Gibbs Symposium | year = 1990 | page = 255}}</ref> Otherwise very little is known of his religious or political views, which he kept to himself.<ref name="Wheeler-views" /> |
||
Gibbs did not produce a substantial personal correspondence and many of his letters were later lost or destroyed.<ref>Rukeyser 1988, pp. 254, 345, 430</ref> Beyond the technical writings concerning his scientific research, he published only two other pieces: a brief obituary for Rudolf Clausius, one of the founders of the mathematical theory of thermodynamics, and a longer biographical memoir of his mentor at Yale, H. A. Newton.<ref>Wheeler 1998, p. 95. See also the ''Collected Works'', vol. II</ref> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | {{quote|was always neatly dressed, usually wore a felt hat on the street, and never exhibited any of the physical mannerisms or eccentricities sometimes thought to be inseparable from genius |
||
| ⚫ | {{quote|Gibbs was not an advertiser for personal renown nor a propagandist for science; he was a scholar, scion of an old scholarly family, living before the days when research had become ''ré''search ... Gibbs was not a freak, he had no striking ways, he was a kindly dignified gentleman.| E. B. Wilson, 1931<ref name="Wilson-reminiscences " />}} |
||
| ⚫ | Gibbs was a careful investor and financial manager, and at his death in 1903 his estate was valued at $100,000.<ref name="Seeger-gentleman" /> He served for many years as trustee, secretary, and treasurer of his alma mater, the |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | {{quote|was always neatly dressed, usually wore a felt hat on the street, and never exhibited any of the physical mannerisms or eccentricities sometimes thought to be inseparable from genius ... His manner was cordial without being effusive and conveyed clearly the innate simplicity and sincerity of his nature.| Lynde Wheeler, 1951<ref name="Wheeler-portrait" />}} |
||
| ⚫ | {{quote|Gibbs was not an advertiser for personal renown nor a propagandist for science; he was a scholar, scion of an old scholarly family, living before the days when research had become ''ré''search |
||
| ⚫ | Gibbs was a careful investor and financial manager, and at his death in 1903 his estate was valued at $100,000.<ref name="Seeger-gentleman" /> He served for many years as trustee, secretary, and treasurer of his alma mater, the Hopkins School.<ref name="Wheeler-HopkinsSchool">Wheeler, 1998, p. 144</ref> US President [[Chester A. Arthur]] appointed him as one of the commissioners to the National Conference of Electricians, which convened in [[Philadelphia]] in September 1884, and Gibbs presided over one of its sessions.<ref name="Seeger-gentleman" /> |
||
In an obituary published in the ''[[American Journal of Science]]'', Gibbs's former student [[Henry Andrews Bumstead|Henry A. Bumstead]] referred to Gibbs's personal character: |
In an obituary published in the ''[[American Journal of Science]]'', Gibbs's former student [[Henry Andrews Bumstead|Henry A. Bumstead]] referred to Gibbs's personal character: |
||
| Line 107: | Line 114: | ||
== Major scientific contributions == |
== Major scientific contributions == |
||
=== Chemical thermodynamics === |
=== Chemical thermodynamics === |
||
[[File:Wykres Gibbsa.svg|thumb|200px|upright|right| |
[[File:Wykres Gibbsa.svg|thumb|200px|upright|right|Graph of the thermodynamic free energy, showing a plane perpendicular to the axis of ''v'' (volume) and passing through point ''A'' representing the body's initial state. ''MN'' is the section of the "surface of dissipated energy". Qε and Qη are sections of the planes ''η'' = 0 and ''ε'' = 0, and therefore parallel to the axes of ε (internal energy) and η (entropy) respectively. ''AD'' and ''AE'' are, respectively, the energy and entropy of the body in its initial state; ''AB'' and ''AC'' its "available energy" ([[Helmholtz free energy]]) and its "capacity for entropy" (the amount by which the entropy of the body can be increased without changing the energy of the body or increasing its volume). The figure appears in Gibbs's paper from 1873.]] |
||
Gibbs's papers from the 1870s introduced the idea of expressing the internal energy |
Gibbs's papers from the 1870s introduced the idea of expressing the internal energy ''U'' of a system in terms of the [[entropy]] ''S'', in addition to the usual [[state variable|state-variable]]s of volume ''V'', pressure ''p'', and temperature ''T''.<ref name="Klein-bio" /> He also introduced the concept of the [[chemical potential]] <math>\mu</math> of a given chemical species, defined to be the rate of the increase in ''U'' associated with the increase in the number ''N'' of molecules of that species (at constant entropy and volume). Thus, it was Gibbs who first combined the first and second [[laws of thermodynamics]]<ref name="Klein-bio" /> by expressing the infinitesimal change in the energy a system in the form: |
||
<math>\mathrm{d}U = T\mathrm{d}S - p \,\mathrm{d}V + \sum_i \mu_i \,\mathrm{d} N_i\,</math> |
<math>\mathrm{d}U = T\mathrm{d}S - p \,\mathrm{d}V + \sum_i \mu_i \,\mathrm{d} N_i\,</math> |
||
where the sum in the last term is over the different chemical species. By taking the [[Legendre transformation|Legendre transform]] of this expression, he defined the concepts of [[enthalpy]] and "free energy" (now universally known as the "[[Gibbs free energy]]"), a [[thermodynamic potential]] which is especially useful to chemists since it determines whether a reaction will proceed spontaneously at a fixed temperature and pressure. In the same way he also obtained what is now known as the "[[Gibbs–Duhem equation]] |
where the sum in the last term is over the different chemical species. By taking the [[Legendre transformation|Legendre transform]] of this expression, he defined the concepts of [[enthalpy]] and "free energy" (now universally known as the "[[Gibbs free energy]]"), a [[thermodynamic potential]] which is especially useful to chemists since it determines whether a reaction will proceed spontaneously at a fixed temperature and pressure. In the same way he also obtained what is now known as the "[[Gibbs–Duhem equation]]".<ref name="Wheeler-thermodynamics" /><ref name="Klein-bio" /> |
||
The publication of the paper " |
The publication of the paper "On the Equilibrium of Heterogeneous Substances" (1874–78) is now regarded as a landmark in the development of physical chemistry. That paper formulated the [[Gibbs phase rule|phase rule]] for the number of [[Intensive and extensive properties|variables]] that can be controlled in a heterogeneous mixture in equilibrium (see also [[phase diagram]]). It also developed a rigorous mathematical theory for various [[transport phenomena]], including [[electrochemistry|electrochemical processes]] and the [[Marangoni effect]] in fluid mixtures.<ref name="Wheeler-thermodynamics" /> |
||
=== |
=== Statistical mechanics === |
||
| ⚫ | |||
| ⚫ | Together with James Clerk Maxwell and [[Ludwig Boltzmann]], Gibbs is considered one of the founders of statistical mechanics. It was Gibbs who coined the term "statistical mechanics" to identify the branch of theoretical physics that accounts for the observed thermodynamic properties of systems in terms of the statistics of large ensembles of particles. He introduced the concept of [[phase space]] and used it to define the [[microcanonical ensemble|microcanonical]], [[canonical ensemble|canonical]], and [[grand canonical ensemble]]s, thus obtaining a more general formulation of the statistical properties of many-particle systems than what Maxwell and Boltzmann had achieved before.<ref name="Klein-bio" /><ref name="Wheeler-statistical" /> |
||
| ⚫ | |||
According to [[Henri Poincaré]], writing in 1904, even though Maxwell and Boltzmann had previously explained the [[Irreversible process|irreversibility]] of macroscopic physical processes in probabilistic terms, "the one who has seen it most clearly, in a book too little read because it is a little difficult to read, is Gibbs, in his ''Elementary Principles of Statistical Mechanics''."<ref name="Poincare">{{Citation|last=Poincaré |first=Henri |authorlink=Henri Poincaré |year=1904 |chapter=[[s:The Principles of Mathematical Physics|The Principles of Mathematical Physics]]|title=The Foundations of Science (The Value of Science)|pages=297–320|publisher=Science Press|place=New York}}</ref> Gibbs's analysis of irreversibility, and his formulation of the [[H-theorem]] and the [[ergodic hypothesis]], were major influences in the mathematical physics of the 20th century.<ref name="Wiener">{{cite book | last = Wiener | first = Norbert | title =Cybernetics: or Control and Communication in the Animal and the Machine | chapter = II: Groups and Statistical Mechanics | edition = 2| publisher = MIT Press | year = 1961 | isbn = 978-0-262-23007-0}}</ref><ref name="Wightman">{{cite book | chapter = On the Prescience of J. Willard Gibbs | last = Wightman | first = Arthur S. | title = Proceedings of the Gibbs Symposium | year = 1990 |pages = 23–38}}</ref> |
|||
=== Statistical mechanics === |
|||
| ⚫ | Gibbs was well aware that the application of the [[equipartition theorem]] to large systems of classical particles failed to explain the measurements of the [[Heat capacity|specific heats]] of both solids and gases, and he argued that this was evidence of the danger of basing thermodynamics on "hypotheses about the constitution of matter".<ref name="Wheeler-statistical" /> Gibbs's own framework for statistical mechanics was so carefully constructed that it could be carried over almost intact after the discovery that the microscopic laws of nature obey the rules of [[quantum mechanics]], rather than the classical mechanics known to Gibbs and to his contemporaries.<ref name="MacTutor" /> His resolution of the so-called "[[Gibbs paradox]]," about the entropy of the mixing of gases, is now often cited as a prefiguration of the [[Identical particles|indistinguishability of particles]] required by quantum mechanics.<ref>See, e.g., {{cite book | last = Huang | first = Kerson | authorlink = Kerson Huang | title = Statistical Mechanics | publisher = John Wiley & Sons | year = 1987 | edition = 2 | pages = 140–143 | isbn = 0-471-81518-7}}</ref> |
||
=== Vector analysis === |
|||
| ⚫ | |||
British scientists, including Maxwell, had relied on Hamilton's [[quaternion]]s in order to express the dynamics of physical quantities, like the electric and magnetic fields, having both a magnitude and a direction in three-dimensional space. Gibbs, however, noted that the product of quaternions always had to be separated into two parts: a one-dimensional (scalar) quantity and a three-dimensional [[Euclidean vector|vector]], so that the use of quaternions introduced mathematical complications and redundancies that could be avoided in the interest of simplicity and to facilitate teaching. He therefore proposed defining distinct [[dot product|dot]] and [[cross product]]s for pairs of vectors and introduced the now common notation for them. He was also largely responsible for the development of the [[vector calculus]] techniques still used today in electrodynamics and fluid mechanics. In this effort, Gibbs was guided by the earlier work of Grassmann on "[[exterior algebra]]".<ref>Wheeler 1998, p. 109</ref> |
|||
| ⚫ | Together with |
||
| ⚫ | As Gibbs had advocated in the 1880s and 1890s, quaternions were eventually all but abandoned by physicists in favor of the vectorial approach developed by him and, independently, by Oliver Heaviside. Gibbs applied his vector methods to the determination of planetary and comet [[orbit]]s. He also developed the concept of mutually reciprocal triads of vectors that later proved to be of importance in [[crystallography]].<ref>{{cite book | chapter = Reciprocal Space in Crystallography | last = Shmueli | first = Uri | title = International Tables for Crystallography | volume = B | year = 2006 |pages = 2–9 | url = http://www.mendeley.com/research/11-reciprocal-space-crystallography/#page-1}}</ref> |
||
| ⚫ | Gibbs was well aware that the application of the [[equipartition theorem]] to large systems of classical particles failed to explain the measurements of the [[Heat capacity|specific heats]] of both solids and gases, and he argued that this was evidence of the danger of basing thermodynamics on "hypotheses about the constitution of matter |
||
=== Physical optics === |
=== Physical optics === |
||
| Line 135: | Line 147: | ||
== Scientific recognition == |
== Scientific recognition == |
||
Gibbs worked at a time when there was little tradition of rigorous theoretical science in the United States. His research was not easily understandable to his students or his colleagues and he made no effort to popularize his ideas or to simplify their exposition to make them more accessible.<ref name="MacTutor" /> His seminal work on thermodynamics was published mostly in the ''Transactions of the Connecticut Academy'', a journal edited by his librarian brother-in-law, which was little read in the USA and even less so in Europe. When Gibbs submitted his long paper on the equilibrium of heterogeneous substances to the Academy, both [[Elias Loomis]] and |
Gibbs worked at a time when there was little tradition of rigorous theoretical science in the United States. His research was not easily understandable to his students or his colleagues and he made no effort to popularize his ideas or to simplify their exposition to make them more accessible.<ref name="MacTutor" /> His seminal work on thermodynamics was published mostly in the ''Transactions of the Connecticut Academy'', a journal edited by his librarian brother-in-law, which was little read in the USA and even less so in Europe. When Gibbs submitted his long paper on the equilibrium of heterogeneous substances to the Academy, both [[Elias Loomis]] and Hubert Anson Newton protested that they did not understand Gibbs's work at all, but they helped to raise the money needed to pay for the typesetting of the many equations and mathematical symbols in the paper. Funds for the purpose were contributed both by members of the university and by local business and professional men in New Haven.<ref name="Rukeyser-printing">Rukeyser 1998, pp. 225–6</ref> |
||
According to James Gerald Crowther, |
According to James Gerald Crowther, |
||
| Line 141: | Line 153: | ||
{{quote|in his later years [Gibbs] was a tall, dignified gentleman, with a healthy stride and ruddy complexion, performing his share of household chores, approachable and kind (if unintelligible) to students. Gibbs was highly esteemed by his friends, but American science was too preoccupied with practical questions to make much use of his profound theoretical work during his lifetime. He lived out his quiet life at Yale, deeply admired by a few able students but making no immediate impress on American science commensurate with his genius.| J. G. Crowther, 1937<ref name="MacTutor" />}} |
{{quote|in his later years [Gibbs] was a tall, dignified gentleman, with a healthy stride and ruddy complexion, performing his share of household chores, approachable and kind (if unintelligible) to students. Gibbs was highly esteemed by his friends, but American science was too preoccupied with practical questions to make much use of his profound theoretical work during his lifetime. He lived out his quiet life at Yale, deeply admired by a few able students but making no immediate impress on American science commensurate with his genius.| J. G. Crowther, 1937<ref name="MacTutor" />}} |
||
In his autobiography, mathematician [[Gian-Carlo Rota]] tells of casually browsing the mathematical stacks of [[Sterling Library]] and stumbling on a handwritten mailing list, attached to some of Gibbs's course notes, which listed over two hundred notable scientists of his day, including |
In his autobiography, mathematician [[Gian-Carlo Rota]] tells of casually browsing the mathematical stacks of [[Sterling Library]] and stumbling on a handwritten mailing list, attached to some of Gibbs's course notes, which listed over two hundred notable scientists of his day, including Poincaré, Boltzmann, [[David Hilbert]], and [[Ernst Mach]].<ref name="Rota">{{cite book | last = Rota | first = Gian-Carlo | authorlink=Gian-Carlo Rota | title = Indiscrete Thoughts | publisher = Birkhäuser | year = 1996 | page = 25 |isbn = 978-0-8176-3866-5}}</ref> (Lynde Wheeler reproduces this mailing list in an appendix to his biography of Gibbs.<ref name="Wheeler-mailing">Wheeler 1998, appendix IV</ref>) One may conclude that Gibbs's work was better known among the scientific elite of his day than the published material suggests.<ref name="Rota" /> Gibbs succeeded in interesting his European correspondents in that work, which was translated into German (then the leading language for chemistry) by [[Wilhelm Ostwald]] in 1892 and into French by [[Henri Louis Le Châtelier]] in 1899. |
||
Gibbs |
Gibbs received the major honors then possible for an academic scientist in the US: he was elected to the [[National Academy of Sciences]] in 1879 and was awarded the 1880 [[Rumford Prize]] from the [[American Academy of Arts and Sciences]] for his work on chemical thermodynamics.<ref>{{cite book | last = Müller | first = Ingo | title = A History of Thermodynamics - the Doctrine of Energy and Entropy | publisher = Springer | year = 2007 | isbn = 978-3-540-46226-2}}</ref> He was also granted honorary doctorates from Princeton University and [[Williams College]] in the US, and from the universities of [[University of Erlangen-Nuremberg|Erlangen]] and [[University of Oslo|Christiania]] (now Oslo) in Europe.<ref name="Bumstead" /> He was inducted as a foreign member of the [[Royal Society]] of London in 1897 and received the Society's [[Copley Medal]] in 1901.<ref name="MacTutor" /> At the time, that was considered the highest international honor in the natural sciences.<ref name="APS" /> Gibbs was also a corresponding member of the [[Prussian Academy of Sciences|Prussian]] and [[French Academy of Sciences|French]] Academies of Science.<ref name="Bumstead" /> |
||
==Influence== |
==Influence== |
||
Gibbs's most immediate and obvious influence was on physical chemistry and statistical mechanics, two disciplines which he greatly helped to found. During Gibbs's lifetime, his [[Gibbs' phase rule|phase rule]] was experimentally validated by Dutch chemist [[Hendrik Willem Bakhuis Roozeboom|H. W. Bakhuis Roozeboom]], who showed how to apply it in a variety of situations, thereby assuring it of widespread use.<ref name="Roozeboom">{{cite book | last=Crowther | first=James Gerald | chapter=Josiah Willard Gibbs, 1839–1903 | title=Famous American Men of Science | publisher=Books for Libraries | location=Freeport, NY | year=1969 [1937] | pages=277–8}}</ref> In industrial chemistry, Gibbs's thermodynamics found many applications in the early 20th century, from electrochemistry to the development of the [[Haber process]] for the synthesis of [[ammonia]].<ref name="Haber">{{cite doi|10.1016/S0016-0032(25)90344-4}}</ref> |
|||
According to American experimental physicist and Nobel laureate [[Robert Andrews Millikan|Robert A. Millikan]], |
|||
| ⚫ | When Dutch physicist [[Johannes Diderik van der Waals|J. D. van der Waals]] received the 1910 [[Nobel Prize in Physics|Nobel Prize]] "for his work on the [[equation of state]] for gases and liquids" he acknowledged the great influence of Gibbs's work on that subject.<ref name="vanderWaals">{{cite web |url=http://www.nobelprize.org/nobel_prizes/physics/laureates/1910/waals-lecture.html | title = Nobel Lecture: The Equation of State for Gases and Liquids | last =van der Waals | first = J. D. | authorlink = Johannes Diderik van der Waals | year= 1910 |work= Nobel Prize in Physics |publisher = Nobel Foundation}}</ref> [[Max Planck]] received the 1918 Nobel Prize for his work on quantum mechanics, particularly his 1900 paper on the quantization of [[black-body radiation]] (see [[Planck's law]]). That work was based largely on the thermodynamics of Kirchhoff, Boltzmann, and Gibbs. According to Planck, Gibbs's name "not only in America but in the whole world will ever be reckoned among the most renowned theoretical physicists of all times."<ref name="Planck">{{cite book | last=Planck | first=Max |authorlink=Max Planck | title=Eight Lectures on Theoretical Physics | chapter=Second Lecture: Thermodynamic States of Equilibrium in Dilute Solutions | publisher=Columbia University Press | location=New York | year=1915 | page=21 | url=http://books.google.com/books?id=53DnAAAAMAAJ&pg=PA21}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | The first half of the 20th century saw the publication of two influential textbooks that soon came to be regarded as founding documents of [[chemical thermodynamics]], both of which used and extended Gibbs's work in that field: these were ''Thermodynamics and the Free Energy of Chemical Processes'' (1923), by [[Gilbert N. Lewis]] and [[Merle Randall]], and ''Modern Thermodynamics by the Methods of Willard Gibbs'' (1933), by [[Edward A. Guggenheim]].<ref name="Ott">{{cite book | last = Ott | first = Bevan J. | coauthors = Boerio-Goates, Juliana | title = Chemical Thermodynamics – Principles and Applications | publisher = Academic Press | year = 2000 | isbn = 0-12-530990-2}}</ref> Under the influence of Lewis, [[William Giauque]] (who had originally wanted to be a [[chemical engineer]]) went on to become a professor of chemistry at [[University of California, Berkeley|Berkeley]] and won the 1949 [[Nobel Prize in Chemistry]] for his investigations into the properties of matter at temperatures close to absolute zero, studies guided by the [[third law of thermodynamics]].<ref name="Giauque">{{cite web |url=http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1949/press.html |title=Award Ceremony Speech | last = Tiselius | first = Arne | authorlink = Arne Tiselius |year= 1949 |work= Nobel Prize in Chemistry |publisher= Nobel Foundation}}</ref> |
||
| ⚫ | |||
| ⚫ | Gibbs's early papers on the use of graphical methods in thermodynamics reflect a powerfully original understanding of what mathematicians would later call [[convex analysis]], including ideas that, according to [[Barry Simon]], "lay dormant for about seventy-five years".<ref>{{cite book | last = Simon | first = Barry | authorlink = Barry Simon | title =Convexity: An Analytic Viewpoint | publisher = Cambridge University Press | year = 2011 | page = 287 | isbn = 1-1070-0731-3}}</ref> Gibbs's later work on statistical ensembles, as presented in his 1902 textbook, has had an even greater impact in both theoretical physics and in pure mathematics.<ref name="Wiener" /><ref name="Wightman" /> Initially unaware of Gibbs's contributions in that field, [[Albert Einstein]] wrote three papers on statistical mechanics, published between 1902 and 1904. After reading Gibbs's textbook (which was translated into German by [[Ernst Zermelo]] in 1905), Einstein declared that Gibbs's treatment was superior to his own and explained that he would not have written those papers if he had known Gibbs's work.<ref name="Navarro-Einstein">{{cite journal | last=Navarro | first= Luis | year=1998 |title = Gibbs, Einstein and the Foundations of Statistical Mechanics |journal = Archive for History of Exact Sciences |volume = 53 |pages=147–180| url = http://wien.jhu.edu/AnnusMirabilis/AeReserveArticles/Navarro.pdf | doi=10.1007/s004070050025 | issue=2}}</ref> According to mathematical physicist [[Arthur Wightman]]: |
||
| ⚫ | The first half of the 20th century saw the publication of two influential textbooks that soon came to be regarded as founding documents of [[chemical thermodynamics]], both of which used and extended Gibbs's work in that field: these were ''Thermodynamics and the Free Energy of Chemical Processes'' (1923), by [[Gilbert N. Lewis]] and [[Merle Randall]], and ''Modern Thermodynamics by the Methods of Willard Gibbs'' (1933), by [[Edward A. Guggenheim]].<ref name="Ott">{{cite book | last = Ott | first = Bevan J. | coauthors = Boerio-Goates, Juliana | title = Chemical Thermodynamics – Principles and Applications | publisher = Academic Press | year = 2000 | isbn = 0-12-530990-2}}</ref> Under the influence of Lewis, [[William Giauque]] (who had originally wanted to be a [[chemical engineer]]) went on to become a professor of chemistry at [[University of California, Berkeley|Berkeley]] and won the 1949 [[Nobel Prize in Chemistry]] for his investigations into the properties of matter at temperatures close to absolute zero, studies guided by the [[third law of thermodynamics]].<ref name="Giauque">{{cite web |url=http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1949/press.html |title=Award Ceremony Speech | |
||
| ⚫ | {{quote|It is one of the striking features of the work of Gibbs, noticed by every student of thermodynamics and statistical mechanics, that his formulations of physical concepts were so felicitously chosen that they have survived 100 years of turbulent development in theoretical physics and mathematics.| A. S. Wightman, 1990<ref name="Wightman" />}} |
||
| ⚫ | Gibbs's |
||
| ⚫ | The development of vector calculus was Gibbs's other great contribution to mathematics. The publication in 1901 of E. B. Wilson's textbook ''Vector Analysis'', based on Gibbs's lectures at Yale, did much to propagate the use of vectorial methods and notation in both mathematics and theoretical physics, definitively displacing the quaternions that had until then been dominant in the scientific literature.<ref name="Marsden">{{cite book | last1 = Marsden | first1 = Jerrold E. | authorlink1= Jerrold E. Marsden | last2 = Tromba | first2 = Anthony J. | title = Vector Calculus | edition = 3 | publisher = W. H. Freeman | year = 1988 | page = 18 | isbn = 0-7167-1856-1}}</ref> |
||
| ⚫ | {{quote|It is one of the striking features of the work of Gibbs, noticed by every student of thermodynamics and statistical mechanics, that his formulations of physical concepts were so |
||
| ⚫ | At Yale, Gibbs was also the mentor of Lee De Forest, who went on to invent to the [[triode]] amplifier and has been called the "father of radio".<ref name="Seeger-DeForest">Seeger 1974, p. 18</ref> According to De Forest, it was thanks to Gibbs that he realized early on "that the leaders in electrical development would be those who pursued the [[Electromagnetic radiation|higher theory of waves]] and oscillations and the transmission by these means of intelligence and power."<ref name="DeForest" /> Another student of Gibbs who played a significant role in the development of radio technology was Lynde Wheeler. |
||
| ⚫ | The development of |
||
| ⚫ | Gibbs also had an indirect influence on mathematical economics. He supervised the thesis of Irving Fisher, who received the first Ph.D. in economics from Yale in 1891. In that work, published in 1892 as ''Mathematical Investigations in the Theory of Value and Prices'', Fisher drew a direct analogy between Gibbsian equilibrium in physical and chemical systems, and the [[General equilibrium theory|general equilibrium]] of markets, and he used Gibbs's vectorial notation.<ref name="Fisher" /><ref name="Leontief">{{cite journal | last=Leontief |first= Wassily |authorlink=Wassily Leontief |year=1954 |title=Mathematics in economics |journal=Bulletin of the American Mathematical Society |volume=60 |issue=3 |pages=215–233| url=http://projecteuclid.org/euclid.bams/1183518813 | doi=10.1090/S0002-9904-1954-09791-4}}</ref> Gibbs's protegé Edwin Bidwell Wilson became, in turn, a mentor to leading American economist and Nobel Laureate [[Paul Samuelson]].<ref name="Samuelson-lecture"> |
||
| ⚫ | At Yale, Gibbs was also the mentor of |
||
{{cite web |url=http://nobelprize.org/nobel_prizes/economics/laureates/1970/samuelson-lecture.pdf | title=Maximum Principles in Analytical Economics | last = Samuelson | first = Paul A. |year= 1970 |work= Nobel Prize Lecture | publisher= Nobel Foundation}}</ref> In 1947, Samuelson published ''[[Foundations of Economic Analysis]]'', based on his doctoral dissertation, in which he used as [[Epigraph (literature)|epigraph]] a remark attributed to Gibbs: "Mathematics is a language." Samuelson explicitly acknowledged the influence of Gibbs's classical thermodynamic methods<ref name="Samuelson-lecture" /> and identified him as "Yale's great physicist".<ref name="Samuelson-article">{{cite web |url=http://www.nobelprize.org/nobel_prizes/economics/laureates/1970/samuelson-article2.html |title=How I Became an Economist | last = Samuelson | first = Paul A. | date= 5 Sep. 2003 |work= Prize in Economic Sciences |publisher= Nobel Foundation |accessdate=16 Jun. 2012}}</ref> |
|||
| ⚫ | For his part, mathematician [[Norbert Wiener]] cited Gibbs's use of probability in the formulation of statistical mechanics as "the first great revolution of twentieth century physics" and as a major influence on his conception of [[cybernetics]]. Wiener explained in the preface to his book ''[[The Human Use of Human Beings]]'' that it was "devoted to the impact of the Gibbsian point of view on modern life, both through the substantive changes it has made to working science, and through the changes it has made indirectly in our attitude to life in general."<ref>{{cite book | last = Wiener | first = Norbert | title =The Human Use of Human Beings: Cybernetics and Society | publisher = Houghton Mifflin| year = 1950 | pages = 10–11}}</ref> |
||
| ⚫ | Gibbs also had an indirect influence on mathematical economics. He supervised the thesis of |
||
| ⚫ | For his part, mathematician [[Norbert Wiener]] cited Gibbs's use of probability in the formulation of statistical mechanics as "the first great revolution of twentieth century physics" and as a major influence on his conception of [[cybernetics]]. Wiener explained in the preface to his book ''[[The Human Use of Human Beings]]'' that it was "devoted to the impact of the Gibbsian point of view on modern life, both through the substantive changes it has made to working science, and through the changes it has made indirectly in our attitude to life in general."<ref>{{cite book | last = Wiener | first = Norbert | title =The Human Use of Human Beings: Cybernetics and Society | publisher = Houghton Mifflin| year = 1950 | pages = |
||
==Commemoration== |
==Commemoration== |
||
[[File:JWGibbs-tablet.jpg|thumb|200px|Bronze memorial tablet |
[[File:JWGibbs-tablet.jpg|thumb|200px|Bronze memorial tablet, originally installed in 1912 at the Sloane Physics Laboratory, now at the entrance to the J. W. Gibbs Laboratories, Yale University]] |
||
When the German physical chemist [[Walther Nernst]] visited Yale in 1906 to give the [[Silliman Memorial Lectures|Silliman lecture]], he was surprised to discover that there was no tangible memorial for Gibbs. He therefore donated his $500 lecture fee to the university to help pay for a suitable monument, which was finally unveiled in 1912 in the form of a bronze bas-relief by sculptor [[Lee Lawrie]], installed in the Sloane Physics Laboratory.<ref name="Seeger-memorial">Seeger 1974, p. 21</ref> In 1910, the [[American Chemical Society]] established the [[Willard Gibbs Medal]], through the initiative of William A. Converse, a former chairman and secretary of the Chicago Section.<ref>{{cite web |url=http://chicagoacs.net/Gibbs_history.html |title=The Willard Gibbs Medal Founded by William A. Converse |author= |date= |work=|publisher=American Chemical Society, Chicago Section |accessdate=16 |
When the German physical chemist [[Walther Nernst]] visited Yale in 1906 to give the [[Silliman Memorial Lectures|Silliman lecture]], he was surprised to discover that there was no tangible memorial for Gibbs. He therefore donated his $500 lecture fee to the university to help pay for a suitable monument, which was finally unveiled in 1912 in the form of a bronze bas-relief by sculptor [[Lee Lawrie]], installed in the Sloane Physics Laboratory.<ref name="Seeger-memorial">Seeger 1974, p. 21</ref> In 1910, the [[American Chemical Society]] established the [[Willard Gibbs Medal]], through the initiative of William A. Converse, a former chairman and secretary of the Chicago Section.<ref>{{cite web |url=http://chicagoacs.net/Gibbs_history.html |title=The Willard Gibbs Medal Founded by William A. Converse |author= |date= |work=|publisher=American Chemical Society, Chicago Section |accessdate=16 Jun. 2012}}</ref> The [[American Mathematical Society]] endowed the Josiah Willard Gibbs Lectureship in 1923 to increase public awareness of mathematics and its applications.<ref>{{cite web |url=http://www.ams.org/meetings/gibbs-lect.html |title=Josiah Willard Gibbs Lectures |author= |date= |work=Special Lectures |publisher=American Mathematical Society |accessdate=16 Jun. 2012}}</ref> |
||
In 1945, Yale University created the J. Willard Gibbs Professorship in Theoretical Chemistry, held until 1973 by [[Lars Onsager]], who won the 1968 Nobel Prize in chemistry. (Onsager, like Gibbs, worked primarily on the application of new mathematical ideas to problems in physical chemistry.) Yale's |
In 1945, Yale University created the J. Willard Gibbs Professorship in Theoretical Chemistry, held until 1973 by [[Lars Onsager]], who won the 1968 Nobel Prize in chemistry. (Onsager, like Gibbs, worked primarily on the application of new mathematical ideas to problems in physical chemistry.) Yale's Josiah Willard Gibbs Laboratories and its J. Willard Gibbs Assistant Professorship in Mathematics are also named in his honor, and the university has hosted two symposia dedicated to Gibbs's life and work, one in 1989 and another on the centenary of his death, in 2003.<ref>{{cite journal |year=2003 |title=Forum News |journal=History of Physics Newsletter |volume=8 |issue=6 |pages=3| url=http://www.aps.org/units/fhp/newsletters/upload/february03.pdf}}</ref> [[Rutgers University]] has a J. Willard Gibbs Professorship of Thermomechanics, presently held by Bernard D. Coleman.<ref>{{cite web |url=http://www.mechanics.rutgers.edu/BDC.html |title=Faculty webpage | last = Coleman | first = Bernard D |date= |work= |publisher=Rutgers University, Dept. of Mechanics and Materials Science |accessdate=16 Jun. 2012}}</ref> |
||
In 1950, Gibbs was elected to the [[Hall of Fame for Great Americans]].<ref name"HallofFame">{{cite web |url=http://www.medalcollectors.org/Guides/HFGA/HFGA.html |title=The Hall of Fame for Great Americans at New York University | |
In 1950, Gibbs was elected to the [[Hall of Fame for Great Americans]].<ref name="HallofFame">{{cite web |url=http://www.medalcollectors.org/Guides/HFGA/HFGA.html |title=The Hall of Fame for Great Americans at New York University | last = Johnson | first = D. Wayne |date= |publisher=Medal Collectors of America |accessdate=16 Jun. 2012}}</ref> The [[United States Navy]] [[oceanographic research ship]] [[USNS Josiah Willard Gibbs (T-AGOR-1)|USNS ''Josiah Willard Gibbs'' (T-AGOR-1)]], in service from 1958 to 1971, was named for Gibbs.<ref name="ship">{{cite web |url=http://www.history.navy.mil/danfs/s4/san_carlos.htm |title=San Carlos |author= |date= |work=Dictionary of American Naval Fighting Ships |publisher=Naval History and Heritage Command |accessdate=16 Jun. 2012}}</ref> |
||
E. A. Guggenheim introduced the symbol ''G'' for the Gibbs free energy in 1933, and the same symbol was used also by [[Dirk ter Haar]] in 1966.<ref name="G">Seeger 1974, p. 96</ref> This notation is now universal and is recommended by the [[International Union of Pure and Applied Chemistry|IUPAC]].<ref name="IUPAC-G">{{cite doi |10.1351/goldbook.G02629}}</ref> In 1960, William Giauque ''et al.'' suggested using the name "gibbs" (abbreviated gbs.) for the unit of entropy, [[calorie]] / [[Kelvin]],<ref name="Giauque-unit">{{cite doi |10.1021/ja01486a014}}</ref> but this usage did not become common and the corresponding [[International System of Units|SI]] unit, [[Joule]] / Kelvin, carries no special name. |
|||
| ⚫ | |||
[[File:JWGibbsLabs.jpg|thumb|left|300px|Building housing the Josiah Willard Gibbs Laboratories, at Yale University's Science Hill]] |
[[File:JWGibbsLabs.jpg|thumb|left|300px|Building housing the Josiah Willard Gibbs Laboratories, at Yale University's Science Hill]] |
||
| ⚫ | On May 4, 2005, the [[United States Postal Service]] issued the ''American Scientists'' commemorative [[postage stamp]] series designed by artist [[Victor Stabin]], depicting Gibbs, [[John von Neumann]], [[Barbara McClintock]], and [[Richard Feynman]].<ref name="stamp-issue">{{cite news |title=Yale scientist featured in new stamp series |url=http://www.yale.edu/opa/arc-ybc/v33.n29/story3.html |newspaper=Yale Bulletin & Calendar |date=20 May 2005 | volume= 33| number=28| accessdate=30 Nov. 2012}}</ref> Kenneth R. Jolls, a professor of chemical engineering at [[Iowa State University]] and an expert on graphical methods in thermodynamics, consulted on the design of the stamp honoring Gibbs.<ref name="Jolls-stamp">{{cite news |title=Iowa State Chemical Engineer Drives Issue of New Stamp Honoring Father of Thermodynamics |url=http://www.eng.iastate.edu/coe/feature/jolls.asp |newspaper=College Feature, Iowa State University, College of Engineering |year=2004 |accessdate=17 Nov. 2012}}</ref><ref name="ISU-stamp">{{cite news |title=ISU professor helps develop postage stamp honoring noted scientist |first= Annette |last= Hacker |url=http://www.public.iastate.edu/~nscentral/news/04/nov/stamp.shtml |newspaper=News Service, Iowa State University |date=11 Nov. 2004 |accessdate=17 Nov. 2012}}</ref><ref name="chem-eng-stamp">{{cite journal |last1= |first1= |last2= |first2= |year=2005 |title=Postal Service Pays Homage to Josiah Willard Gibbs |journal=Chemical Engineering Progress |volume=101 |issue=7 |pages=57}}</ref> The stamp identifies Gibbs as a "thermodynamicist" and features a diagram from the 4th edition of Maxwell's ''Theory of Heat'', published in 1875, which illustrates Gibbs's thermodynamic surface for water.<ref name="ISU-stamp" /><ref name="chem-eng-stamp" /> [[Microprinting]] on the [[Collar (clothing)|collar]] of Gibbs's portrait depicts his original mathematical equation for the change in the energy of a substance in terms of its entropy and the other state variables.<ref name="Spakovszky-stamp">{{cite journal |last=Spakovszky |first= Zoltan |year=2005 |title=Stamp of Authenticity |journal=ASME Mechanical Engineering |volume=128 |issue=4 |pages=7 |url=http://web.mit.edu/16.unified/www/FALL/thermodynamics/figures/MechEng-4-2006-Gibbs.pdf}}</ref> |
||
| ⚫ | |||
<!-- Deleted image removed: [[Image:Gibbs-US-stamp.jpg|thumb|upright|US postage stamp honoring Willard Gibbs, issued in 2005 {{FFDC|1=Gibbs-US-stamp.jpg|log=2012 May 4|date=May 2012}}]] --> |
|||
| ⚫ | On May 4, 2005, the [[United States Postal Service]] issued the ''American Scientists'' commemorative [[postage stamp]] series designed by artist [[Victor Stabin]], depicting Gibbs, [[John von Neumann]], [[Barbara McClintock]], and [[Richard Feynman]].<ref name="stamp-issue"> |
||
===In literature=== |
===In literature=== |
||
In 1909, the American historian and novelist [[Henry Adams]] finished an essay entitled "The Rule of Phase Applied to History |
In 1909, the American historian and novelist [[Henry Adams]] finished an essay entitled "The Rule of Phase Applied to History", in which he sought to apply Gibbs's phase rule and other thermodynamic concepts to a general theory of human history. [[William James]], Henry Bumstead, and others criticized both Adams's tenuous grasp of the scientific concepts that he invoked, as well as the arbitrariness of his application of those concepts as metaphors for the evolution of human thought and society.<ref name="Mindel-Adams">{{Cite jstor|2708401}}</ref> The essay remained unpublished until it appeared posthumously in 1919, in ''The Degradation of the Democratic Dogma'', edited by Henry Adams's younger brother [[Brooks Adams|Brooks]].<ref name="Degradation">{{cite book |title=The Degradation of the Democratic Dogma |last=Adams |first=Henry |authorlink=Henry Adams |coauthors=Adams, Brooks |year=1919 |publisher=Macmillan |location=New York |page= |pages= |url=http://archive.org/details/thedegradationof00adamuoft |accessdate=5 May 2012}}</ref> |
||
In the 1930s, feminist poet [[Muriel Rukeyser]] became fascinated by Willard Gibbs and wrote a long poem about his life and work ("Gibbs |
In the 1930s, feminist poet [[Muriel Rukeyser]] became fascinated by Willard Gibbs and wrote a long poem about his life and work ("Gibbs", included in the collection ''A Turning Wind'', published in 1939), as well as a book-length biography (''Willard Gibbs'', 1942).<ref name="Rukeyser">Rukeyser 1988</ref> According to Rukeyser: |
||
{{quote|Willard Gibbs is the type of the imagination at work in the world. His story is that of an opening up which has had its effect on our lives and our thinking; and, it seems to me, it is the emblem of the naked imagination |
{{quote|Willard Gibbs is the type of the imagination at work in the world. His story is that of an opening up which has had its effect on our lives and our thinking; and, it seems to me, it is the emblem of the naked imagination—which is called abstract and impractical, but whose discoveries can be used by anyone who is interested, in whatever "field"—an imagination which for me, more than that of any other figure in American thought, any poet, or political, or religious figure, stands for imagination at its essential points.| Muriel Rukeyser, 1949<ref>{{cite doi|10.1063/1.3066422}}</ref>}} |
||
Both Gibbs and Rukeyser's biography of him figure prominently in the poetry collection ''True North'' (1997) by [[Stephanie Strickland]].<ref name="Strickland">{{cite book |title=True North |last=Strickland |first=Stephanie |authorlink=Stephanie Strickland |year=1997 |publisher=University of Notre Dame Press |location=Notre Dame, IN |isbn= 978- |
Both Gibbs and Rukeyser's biography of him figure prominently in the poetry collection ''True North'' (1997) by [[Stephanie Strickland]].<ref name="Strickland">{{cite book |title=True North |last=Strickland |first=Stephanie |authorlink=Stephanie Strickland |year=1997 |publisher=University of Notre Dame Press |location=Notre Dame, IN |isbn= 978-0-268-01899-3}}</ref> |
||
Gibbs's nephew, Ralph Gibbs Van Name, a professor of physical chemistry at Yale, was unhappy with Rukeyser's biography, in part because of her lack of scientific training. |
Gibbs's nephew, Ralph Gibbs Van Name, a professor of physical chemistry at Yale, was unhappy with Rukeyser's biography, in part because of her lack of scientific training. Van Name had withheld the family papers from her and, after her book was published in 1942 to positive literary but mixed scientific reviews, he tried to encourage Gibbs's former students to produce a new and more technically oriented biography.<ref name="family-papers">{{cite web |url=http://drs.library.yale.edu:8083/HLTransformer/HLTransServlet?stylename=yul.ead2002.xhtml.xsl&pid=beinecke:gibbs&query=&clear-stylesheet-cache=yes&hlon=yes&big=&adv=&filter=&hitPageStart=&sortFields=&view=all |title=Guide to the Gibbs-Van Name Papers |last = Holeman | first = Heather L. |year = 1986 | publisher=Yale University Library |accessdate = 18 Nov. 2012}}</ref> With his support, Lynde Wheeler published such a work in 1951.<ref name="Wheeler">Wheeler 1998</ref> |
||
In fiction, Gibbs appears as the mentor to character Kit Traverse in [[Thomas Pynchon]]'s novel ''[[Against the Day]]'' (2006). That novel also prominently discusses the |
In fiction, Gibbs appears as the mentor to character Kit Traverse in [[Thomas Pynchon]]'s novel ''[[Against the Day]]'' (2006). That novel also prominently discusses the birefringence of [[Iceland spar]], an optical phenomenon that Gibbs investigated.<ref name="Pynchon">{{cite book |title=Against the Day |last=Pynchon |first=Thomas |authorlink=Thomas Pynchon |year=2006 |publisher=Penguin |location=New York |isbn= 978-1-59420-120-2}}</ref> |
||
== Outline of principal work == |
|||
| ⚫ | |||
| ⚫ | |||
* '''[[Statistical mechanics]]''': [[Statistical ensemble (mathematical physics)|statistical ensemble]], [[phase space]], [[Entropy (statistical thermodynamics)#Gibbs Entropy Formula|Gibbs entropy]], [[ergodic hypothesis]], [[Gibbs paradox]] |
* '''[[Statistical mechanics]]''': [[Statistical ensemble (mathematical physics)|statistical ensemble]], [[phase space]], [[Entropy (statistical thermodynamics)#Gibbs Entropy Formula|Gibbs entropy]], [[ergodic hypothesis]], [[Gibbs paradox]] |
||
| Line 206: | Line 214: | ||
* '''[[Electromagnetism]]''': [[Maxwell's equations]], [[birefringence]] |
* '''[[Electromagnetism]]''': [[Maxwell's equations]], [[birefringence]] |
||
* '''[[Mathematics]]''': [[Vector Analysis (Gibbs/Wilson)]], [[cross product]], [[Gibbs phenomenon]] |
* '''[[Mathematics]]''': [[Vector Analysis (Gibbs/Wilson)]], [[cross product]], [[Gibbs phenomenon]] |
||
* '''Named for Gibbs''': [[Gibbs free energy]], [[Gibbs phase rule]], [[Gibbs inequality]], [[Gibbs-Helmholtz equation]], [[Gibbs algorithm]], [[Gibbs distribution]], [[Gibbs state]], [[Gibbs sampling]], [[Marangoni effect|Gibbs-Marangoni effect]], [[Gibbs-Duhem relation]], [[Gibbs-Donnan effect]] |
|||
| ⚫ | |||
* '''Lists''': [[List of physicists]], [[Timeline of thermodynamics, statistical mechanics, and random processes|Timeline of thermodynamics]], [[List of physics topics]], [[List of notable textbooks in statistical mechanics]] |
|||
* [[Timeline of thermodynamics]] |
|||
* [[List of notable textbooks in statistical mechanics]] |
|||
* [[List of theoretical physicists]] |
|||
== References == |
== References == |
||
| Line 213: | Line 225: | ||
== Bibliography == |
== Bibliography == |
||
=== Primary === |
=== Primary === |
||
* L. P. Wheeler, E. O. Waters and S. W. Dudley |
* ''The Early Work of Willard Gibbs in Applied Mechanics'', eds. L. P. Wheeler, E. O. Waters and S. W. Dudley, (New York: Henry Schuman, 1947). ISBN 1-881987-17-5 |
||
* J. W. Gibbs, "[[On the Equilibrium of Heterogeneous Substances]] |
* J. W. Gibbs, "[[On the Equilibrium of Heterogeneous Substances]]", ''Transactions of the Connecticut Academy of Arts and Sciences'', '''3''', 108–248, 343–524, (1874–1878). Reproduced in both the ''Scientific Papers'' (1906) and the ''Collected Works of J. Willard Gibbs'' (1928). |
||
| ⚫ | |||
* [[Edwin Bidwell Wilson|E. B. Wilson]], [http://www.archive.org/details/117714283 ''Vector Analysis, a text-book for the use of students of Mathematics and Physics, founded upon the Lectures of J. Willard Gibbs''], (New Haven: Yale University Press, 1929 [1901]). |
* [[Edwin Bidwell Wilson|E. B. Wilson]], [http://www.archive.org/details/117714283 ''Vector Analysis, a text-book for the use of students of Mathematics and Physics, founded upon the Lectures of J. Willard Gibbs''], (New Haven: Yale University Press, 1929 [1901]). |
||
| ⚫ | |||
* ''The Scientific Papers of J. Willard Gibbs,'' in two volumes, eds. H. A. Bumstead and R. G. Van Name, (Woodbridge, CT: Ox Bow Press, 1993 [1906]). ISBN 0-918024-77-3, ISBN 1-881987-06-X |
* ''The Scientific Papers of J. Willard Gibbs,'' in two volumes, eds. H. A. Bumstead and R. G. Van Name, (Woodbridge, CT: Ox Bow Press, 1993 [1906]). ISBN 0-918024-77-3, ISBN 1-881987-06-X. For scans of the 1906 printing, see [http://archive.org/stream/scientificpapers01gibbuoft#page/n5/mode/2up vol. I] and [http://archive.org/stream/scientificpapers02gibbuoft#page/n3/mode/2up vol. II]. |
||
* ''The Collected Works of J. Willard Gibbs'', in two volumes, eds. W. R. Longley and R. G. Van Name, (New Haven: Yale University Press, 1957 [1928]). |
* ''The Collected Works of J. Willard Gibbs'', in two volumes, eds. W. R. Longley and R. G. Van Name, (New Haven: Yale University Press, 1957 [1928]). |
||
=== Secondary === |
=== Secondary === |
||
* [http://www-gap.dcs.st-and.ac.uk/~history/References/Gibbs.html |
* [http://www-gap.dcs.st-and.ac.uk/~history/References/Gibbs.html References for J Willard Gibbs], ''The MacTutor History of Mathematics archive'', [[University of St Andrews]], School of Mathematics and Statistics (1997). Retrieved 3 Dec. 2012. |
||
* [[Henry Andrews Bumstead|H. A. Bumstead]], "Josiah Willard Gibbs |
* [[Henry Andrews Bumstead|H. A. Bumstead]], "Josiah Willard Gibbs", ''American Journal of Science'', '''16''', 187–202 (1903), reprinted with some additions in both the ''Scientific Papers'' (1906) and the ''Collected Works of J. Willard Gibbs'' (1928), pp. xiii–xxviiii. |
||
* |
* ''Proceedings of the Gibbs Symposium, Yale University, May 15–17, 1989'', eds. D. G. Caldi and G. D. Mostow, (American Mathematical Society and American Institute of Physics, 1990). |
||
* M. J. Crowe, ''A History of Vector Analysis: The Evolution of the Idea of a Vectorial System'', (New York: Dover, 1994 [1967]). ISBN 0- |
* M. J. Crowe, ''A History of Vector Analysis: The Evolution of the Idea of a Vectorial System'', (New York: Dover, 1994 [1967]). ISBN 0-486-67910-1 |
||
* J. G. Crowther, ''Famous American Men of Science'', (Freeport, NY: Books for Libraries Press, 1969 [1937]). ISBN 0-8369-0040-5 |
* J. G. Crowther, ''Famous American Men of Science'', (Freeport, NY: Books for Libraries Press, 1969 [1937]). ISBN 0-8369-0040-5 |
||
* |
* ''A Commentary on the Scientific Writings of J. Willard Gibbs'', in two volumes, eds. F. G. Donnan and A. E. Hass, (New York: Arno, 1980 [1936]). ISBN 0-405-12544-5 |
||
* [[Pierre Duhem|P. Duhem]], [http://archive.org/details/josiahwillardgib00duheuoft ''Josiah-Willard Gibbs à propos de la publication de ses Mémoires scientifiques''], (Paris: A. Herman, 1908). |
* [[Pierre Duhem|P. Duhem]], [http://archive.org/details/josiahwillardgib00duheuoft ''Josiah-Willard Gibbs à propos de la publication de ses Mémoires scientifiques''], (Paris: A. Herman, 1908). |
||
* C. S. Hastings, "Josiah Willard Gibbs |
* C. S. Hastings, [http://archive.org/stream/biographicalmemo011929mbp#page/n417/mode/2up "Josiah Willard Gibbs"], ''Biographical Memoirs of the National Academy of Sciences'', '''6''', 373–393 (1909). |
||
* [[Martin J. Klein|M. J. Klein]], "Gibbs, Josiah Willard |
* [[Martin J. Klein|M. J. Klein]], "Gibbs, Josiah Willard", in ''Complete Dictionary of Scientific Biography'', vol. 5, (Detroit: Charles Scriber's Sons, 2008), pp. 386–93. |
||
* K. Meinke and J. V. Tucker, "Universal Algebra |
* K. Meinke and J. V. Tucker, "Universal Algebra", in ''Handbook of Logic in Computer Science'', vol. I, eds. S. Abramsky, D. Gabbay and T. S. E. Maibaum, (Oxford: Oxford University Press), pp. 189–411. ISBN 0-19-853761-1 |
||
* [[Muriel Rukeyser|M. Rukeyser]], ''Willard Gibbs: American Genius'', (Woodbridge, CT: Ox Bow Press, 1988 [1942]). ISBN 0-918024-57-9 |
* [[Muriel Rukeyser|M. Rukeyser]], ''Willard Gibbs: American Genius'', (Woodbridge, CT: Ox Bow Press, 1988 [1942]). ISBN 0-918024-57-9 |
||
* [[Raymond Seeger|R. J. Seeger]], ''J. Willard Gibbs, American mathematical physicist'' par excellence, (Oxford and New York: Pergamon Press, 1974). ISBN 0-08-018013-2 |
* [[Raymond Seeger|R. J. Seeger]], ''J. Willard Gibbs, American mathematical physicist'' par excellence, (Oxford and New York: Pergamon Press, 1974). ISBN 0-08-018013-2 |
||
* [[Lynde Wheeler|L. P. Wheeler]], ''Josiah Willard Gibbs, The History of a Great Mind'', (Woodbridge, CT: Ox Bow Press, 1998 [1951]). ISBN 1-881987-11-6 |
* [[Lynde Wheeler|L. P. Wheeler]], ''Josiah Willard Gibbs, The History of a Great Mind'', (Woodbridge, CT: Ox Bow Press, 1998 [1951]). ISBN 1-881987-11-6 |
||
* [[Edwin Bidwell Wilson|E. B. Wilson]], [http://projecteuclid.org/euclid.bams/1183494779 "Reminiscences of Gibbs by a student and colleague"], ''Bulletin of the American Mathematical Society'', '''37''', |
* [[Edwin Bidwell Wilson|E. B. Wilson]], [http://projecteuclid.org/euclid.bams/1183494779 "Reminiscences of Gibbs by a student and colleague"], ''Bulletin of the American Mathematical Society'', '''37''', 401–416 (1931). |
||
==External links== |
==External links== |
||
| ⚫ | |||
{{Wikisource-author}} |
{{Wikisource-author}} |
||
* {{MacTutor Biography|id=Gibbs}} |
* {{MacTutor Biography|id=Gibbs}} |
||
| Line 244: | Line 258: | ||
* {{MathGenealogy|id=132578}} |
* {{MathGenealogy|id=132578}} |
||
{{Copley Medallists |
{{Copley Medallists 1901–1950}} |
||
{{Authority control|VIAF=122317710}} |
{{Authority control|VIAF=122317710}} |
||
| ⚫ | |||
{{Persondata<!-- Metadata: see [[Wikipedia:Persondata]] --> |
{{Persondata<!-- Metadata: see [[Wikipedia:Persondata]] --> |
||
|NAME= Gibbs, J. Willard |
|NAME= Gibbs, J. Willard |
||
Revision as of 22:27, 9 December 2012
J. Willard Gibbs | |
|---|---|
 Josiah Willard Gibbs | |
| Born | February 11, 1839 |
| Died | April 28, 1903 (aged 64) New Haven, Connecticut |
| Nationality | United States |
| Alma mater | Yale University |
| Known for | Statistical mechanics Statistical ensemble Phase space Gibbs entropy Ergodic hypothesis Enthalpy Gibbs free energy Gibbs' phase rule Vector calculus Cross product Gibbs phenomenon Gibbs-Helmholtz equation Gibbs-Duhem equation Gibbs algorithm Gibbs distribution Gibbs state Gibbs paradox Gibbs-Thomson effect Gibbs isotherm Gibbs-Donnan effect Gibbs lemma |
| Awards | Rumford Prize (1880), Copley Medal (1901) |
| Scientific career | |
| Fields | Physics, chemistry, mathematics |
| Institutions | Yale University |
| Doctoral advisor | Hubert Anson Newton |
| Doctoral students | Edwin Bidwell Wilson, Irving Fisher, Henry Andrews Bumstead, Lynde Wheeler, Lee De Forest |
| Signature | |
| File:Jwgibbs sig.jpg | |
Josiah Willard Gibbs (February 11, 1839 – April 28, 1903) was an American scientist who made important theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in transforming physical chemistry into a rigorous deductive science. Together with James Clerk Maxwell and Ludwig Boltzmann, he created statistical mechanics (a term that he coined), explaining the laws of thermodynamics as consequences of the statistical properties of large ensembles of particles. Gibbs also worked on the application of Maxwell's equations to problems in physical optics. As a mathematician, he invented modern vector calculus (independently of the British scientist Oliver Heaviside, who carried out similar work during the same period).
In 1863, Yale University awarded Gibbs the first American doctorate in engineering. After a three-year sojourn in Europe, Gibbs spent the rest of his career at Yale, where he was professor of mathematical physics from 1871 until his death. Working in relative isolation, he became the earliest theoretical scientist in the United States to earn an international reputation and was praised by Albert Einstein as "the greatest mind in American history".[1] In 1901 Gibbs received what was then considered the highest honor awarded by the international scientific community, the Copley Medal of the Royal Society of London,[1] "for his contributions to mathematical physics".[2]
Commentators and biographers have remarked on the contrast between Gibbs's quiet, solitary life in turn of the century New England and the great international impact of his ideas. Though his work was almost entirely theoretical, the practical value of Gibbs's contributions became evident with the development of industrial chemistry during the first half of the 20th century. According to Robert A. Millikan, in pure science Gibbs "did for statistical mechanics and for thermodynamics what Laplace did for celestial mechanics and Maxwell did for electrodynamics, namely, made his field a well-nigh finished theoretical structure."[3]
Biography
Family background
Gibbs was the fourth of five children and the only son of Josiah Willard Gibbs and his wife Mary Anna, née Van Cleve. The father was a linguist and theologian who served as professor of sacred literature at Yale Divinity School from 1824 until his death in 1861. He was an active abolitionist and is now chiefly remembered for finding an interpreter for the African passengers of the ship Amistad, allowing them to testify during the trial that followed their rebellion against being sold as slaves.[4]
The younger Willard Gibbs belonged to a long line of American academics and clergymen that stretched back to the 17th century. On his father's side, he was descended from Samuel Willard, who served as acting President of Harvard University from 1701 to 1707. On his mother's side, one of his ancestors was the Rev. Jonathan Dickinson, who was the first president of the College of New Jersey (later Princeton University). His given name, which he shared with his father and several other members of his extended family, derived from his ancestor Josiah Willard, who had been Secretary of the Province of Massachusetts Bay in the 18th century.[5]
Early years

Gibbs was educated at the Hopkins School and entered Yale College in 1854, aged 15. He graduated in 1858 near the top of his class, and was awarded prizes for excellence in mathematics and Latin.[6] He remained at Yale as a graduate student at the Sheffield Scientific School. At the age of 19, soon after his graduation from college, Gibbs was inducted into the Connecticut Academy of Arts and Sciences, a scholarly institution composed primarily of members of the Yale faculty.[7]
Relatively few documents from the period survive and it is impossible to reconstruct the details of Gibbs's early career with precision.[8][9] In the opinion of biographers, Gibbs's principal mentor and champion, in both the University and the Connecticut Academy, was probably the astronomer and mathematician Hubert Anson Newton, a leading authority on meteors, who remained Gibbs's lifelong friend and confidant.[7][9] After the death of his father in 1861, Gibbs inherited enough money to make him financially independent.[10] He suffered from recurrent pulmonary trouble as a young man and his doctors were concerned that he might be susceptible to tuberculosis, which had killed his mother. These problems and a defect in his eyesight probably explain why he did not volunteer to fight in the Civil War of 1861–65.[11] He was not conscripted and he remained at Yale for the duration of the war.[12]
In 1863, Gibbs received the first Ph.D. degree in engineering granted in the US, for a thesis entitled "On the Form of the Teeth of Wheels in Spur Gearing", in which he used geometrical techniques to investigate the optimum design for gears.[13] This was also the fifth Ph.D. granted in the US in any subject.[13] After graduation, Gibbs was appointed as tutor at the College for a term of three years. During the first two years he taught Latin and during the third Natural Philosophy (i.e., physics).[5] In 1866 he patented a design for a railway brake[14] and read a paper before the Connecticut Academy, entitled "The Proper Magnitude of the Units of Length", in which he proposed a scheme for rationalizing the system of units of measurement used in mechanics.[15]
After his term as tutor ended, Gibbs travelled to Europe with his sisters, spending the winter of 1866–67 in Paris, where he attended lectures at the Sorbonne and the Collège de France. From there he went to Berlin, where he attended the lectures of Heinrich Gustav Magnus, and to Heidelberg, where he was exposed to the scientific work of Gustav Kirchhoff and Hermann von Helmholtz. At the time, German academics were the leading authorities in chemistry, thermodynamics, and natural science in general.[17]
Gibbs returned to Yale in June 1869 and briefly taught French to engineering students.[18] It was probably also around this time that he worked on a new design for a steam-engine governor, his last significant investigation in mechanical engineering.[19][20] In 1871 he was appointed Professor of Mathematical Physics at Yale, the first such professorship in the United States. Gibbs, who had independent means and had yet to publish anything, was assigned to teach graduate students exclusively and was hired without salary.[21] Unsalaried teaching positions were common in German universities, on which the system of graduate scientific instruction at Yale was then being modeled.[22]
Middle years

Gibbs published his first work in 1873, at the unusually advanced age of 34.[6] His paper on the geometric representation of thermodynamic quantities appeared in the Transactions of the Connecticut Academy. This journal had few readers capable of understanding Gibbs's work, but he shared reprints with his correspondents in Europe and received a very favorable response from James Clerk Maxwell, at the University of Cambridge. Maxwell even made three plaster casts illustrating Gibbs's construct with his own hands and mailed one to Gibbs. That model is on permanent display at the Yale physics department.[23]
Maxwell included a new chapter on Gibbs's work in the next edition of his Theory of Heat, published in 1875. He explained the usefulness of Gibbs's graphical methods in a lecture to the Chemical Society of London and even referred to it in the article on "Diagrams" that he wrote for the Encyclopædia Britannica.[24] Maxwell's early death in 1879, at the age of 48, precluded further collaboration between him and Gibbs. The joke later circulated in New Haven that "only one man lived who could understand Gibbs's papers. That was Maxwell, and now he is dead."[25]
Gibbs extended his thermodynamic analysis to multi-phase chemical systems (i.e., to systems composed of more than one kind of matter) and considered a variety of concrete applications. He described that research in a long monograph titled "On the Equilibrium of Heterogeneous Substances", published in two parts by the Connecticut Academy between 1874 and 1878. This work begins with a quotation taken from the work of Rudolf Clausius that expresses what would later be called the first and second laws of thermodynamics: "The energy of the world is constant. The entropy of the world tends towards a maximum."[26]
Gibbs's monograph is now deemed to be one of the greatest scientific achievements of the 19th century and one of the foundations of modern physical chemistry.[5] In that work, Gibbs rigorously and ingeniously used his graphic thermodynamic techniques to interpret physico-chemical phenomena, explaining and interrelating what had previously been a mass of isolated facts and observations.[27]
It is universally recognised that its publication was an event of the first importance in the history of chemistry... Nevertheless it was a number of years before its value was generally known, this delay was due largely to the fact that its mathematical form and rigorous deductive processes make it difficult reading for anyone, and especially so for students of experimental chemistry whom it most concerns.
— J. J. O'Connor and E. F. Robertson[6]
Gibbs continued to work without pay until 1880, when the new Johns Hopkins University in Baltimore, Maryland offered him a position paying $3,000 per year. In response, Yale offered him a salary of $2,000, which he was content to accept.[28]
Later years
From 1880 to 1884, Gibbs worked on developing the exterior algebra of Hermann Grassmann into a vector calculus well-suited to the needs of physicists. With this object in mind, Gibbs distinguished between the dot and cross products of two vectors and introduced the concept of dyadics. Similar work was carried out independently, and at around the same time, by the British mathematical physicist and engineer Oliver Heaviside. Gibbs sought to convince other physicists of the convenience of the vectorial approach over the quaternionic calculus of William Rowan Hamilton, which was then widely used by British scientists. This led him, in the early 1890s, to a controversy with Peter Guthrie Tait and others in the pages of Nature.[5]
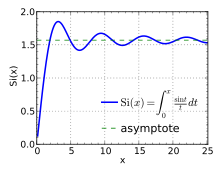
Gibbs's lecture notes on vector calculus were privately printed in 1881 and 1884 for the use of his students, and were later adapted by Edwin Bidwell Wilson into a textbook, Vector Analysis, published in 1901.[5] That book helped to popularize the notation that is widely used today in electrodynamics and fluid mechanics (see del operator). In other mathematical work, he re-discovered the "Gibbs phenomenon" in the theory of Fourier series (which, unbeknownst to him and to later scholars, had been described fifty years before by an obscure English mathematician, Henry Wilbraham).[29]
From 1882 to 1889, Gibbs wrote five papers on physical optics, in which he investigated birefringence and other optical phenomena and defended Maxwell's electromagnetic theory of light against the mechanical theories of Lord Kelvin and others.[5] In his work on optics just as much as in his work on thermodynamics, Gibbs deliberately avoided speculating about the microscopic structure of matter, which proved a wise course in view of the revolutionary developments in quantum mechanics that began around the time of his death.[30]
Gibbs coined the term statistical mechanics and introduced key concepts in the corresponding mathematical description of physical systems, including the notions of chemical potential (1876), statistical ensemble (1878), and phase space (1902).[31][32] Gibbs's derivation of the phenomenological laws of thermodynamics from the statistical properties of systems with many particles was presented in his highly-influential textbook Elementary Principles in Statistical Mechanics, published in 1902, a year before his death.[31]
Gibbs's retiring personality and intense focus on his work limited his accessibility to students. His principal protégé was Edwin Bidwell Wilson, who nonetheless explained that "except in the classroom I saw very little of Gibbs. He had a way, toward the end of the afternoon, of taking a stroll about the streets between his study in the old Sloane Laboratory and his home—a little exercise between work and dinner—and one might occasionally come across him at that time."[33] Gibbs did supervise the doctoral thesis on mathematical economics written by Irving Fisher in 1891.[34] After Gibbs's death, Fisher financed the publication of his Collected Works.[35] Another distinguished student was Lee De Forest, later a pioneer of radio technology.[36]
Gibbs died in New Haven, aged 64, the victim of an acute intestinal obstruction.[33] He is buried in Grove Street Cemetery.[37]
Personal life and character

Gibbs never married, living all his life in his childhood home with his sister Julia and her husband Addison Van Name, who was the Yale librarian. Except for his customary summer vacations in the Adirondacks (at Keene Valley, New York) and later at the White Mountains (in Intervale, New Hampshire),[39] his sojourn in Europe in 1866–69 was almost the only time that Gibbs spent outside New Haven.[5]
Gibbs joined Yale's College Church (a Congregational church) at the end of his freshman year[39][40] and remained a regular attendant for the rest of his life.[41] He generally voted for the Republican candidate in presidential elections, but he supported Grover Cleveland, a conservative Democrat.[42] Otherwise very little is known of his religious or political views, which he kept to himself.[41]
Gibbs did not produce a substantial personal correspondence and many of his letters were later lost or destroyed.[43] Beyond the technical writings concerning his scientific research, he published only two other pieces: a brief obituary for Rudolf Clausius, one of the founders of the mathematical theory of thermodynamics, and a longer biographical memoir of his mentor at Yale, H. A. Newton.[44]
In Edward Bidwell Wilson's view,
Gibbs was not an advertiser for personal renown nor a propagandist for science; he was a scholar, scion of an old scholarly family, living before the days when research had become résearch ... Gibbs was not a freak, he had no striking ways, he was a kindly dignified gentleman.
— E. B. Wilson, 1931[33]
According to Lynde Wheeler, who had been Gibbs's student at Yale, in his later years Gibbs
was always neatly dressed, usually wore a felt hat on the street, and never exhibited any of the physical mannerisms or eccentricities sometimes thought to be inseparable from genius ... His manner was cordial without being effusive and conveyed clearly the innate simplicity and sincerity of his nature.
— Lynde Wheeler, 1951[38]
Gibbs was a careful investor and financial manager, and at his death in 1903 his estate was valued at $100,000.[39] He served for many years as trustee, secretary, and treasurer of his alma mater, the Hopkins School.[45] US President Chester A. Arthur appointed him as one of the commissioners to the National Conference of Electricians, which convened in Philadelphia in September 1884, and Gibbs presided over one of its sessions.[39]
In an obituary published in the American Journal of Science, Gibbs's former student Henry A. Bumstead referred to Gibbs's personal character:
Unassuming in manner, genial and kindly in his intercourse with his fellow-men, never showing impatience or irritation, devoid of personal ambition of the baser sort or of the slightest desire to exalt himself, he went far toward realizing the ideal of the unselfish, Christian gentleman. In the minds of those who knew him, the greatness of his intellectual achievements will never overshadow the beauty and dignity of his life.
— H. A. Bumstead, 1903[5]
Major scientific contributions
Chemical thermodynamics

Gibbs's papers from the 1870s introduced the idea of expressing the internal energy U of a system in terms of the entropy S, in addition to the usual state-variables of volume V, pressure p, and temperature T.[31] He also introduced the concept of the chemical potential of a given chemical species, defined to be the rate of the increase in U associated with the increase in the number N of molecules of that species (at constant entropy and volume). Thus, it was Gibbs who first combined the first and second laws of thermodynamics[31] by expressing the infinitesimal change in the energy a system in the form:
where the sum in the last term is over the different chemical species. By taking the Legendre transform of this expression, he defined the concepts of enthalpy and "free energy" (now universally known as the "Gibbs free energy"), a thermodynamic potential which is especially useful to chemists since it determines whether a reaction will proceed spontaneously at a fixed temperature and pressure. In the same way he also obtained what is now known as the "Gibbs–Duhem equation".[27][31]
The publication of the paper "On the Equilibrium of Heterogeneous Substances" (1874–78) is now regarded as a landmark in the development of physical chemistry. That paper formulated the phase rule for the number of variables that can be controlled in a heterogeneous mixture in equilibrium (see also phase diagram). It also developed a rigorous mathematical theory for various transport phenomena, including electrochemical processes and the Marangoni effect in fluid mixtures.[27]
Statistical mechanics
Together with James Clerk Maxwell and Ludwig Boltzmann, Gibbs is considered one of the founders of statistical mechanics. It was Gibbs who coined the term "statistical mechanics" to identify the branch of theoretical physics that accounts for the observed thermodynamic properties of systems in terms of the statistics of large ensembles of particles. He introduced the concept of phase space and used it to define the microcanonical, canonical, and grand canonical ensembles, thus obtaining a more general formulation of the statistical properties of many-particle systems than what Maxwell and Boltzmann had achieved before.[31][32]
According to Henri Poincaré, writing in 1904, even though Maxwell and Boltzmann had previously explained the irreversibility of macroscopic physical processes in probabilistic terms, "the one who has seen it most clearly, in a book too little read because it is a little difficult to read, is Gibbs, in his Elementary Principles of Statistical Mechanics."[46] Gibbs's analysis of irreversibility, and his formulation of the H-theorem and the ergodic hypothesis, were major influences in the mathematical physics of the 20th century.[47][48]
Gibbs was well aware that the application of the equipartition theorem to large systems of classical particles failed to explain the measurements of the specific heats of both solids and gases, and he argued that this was evidence of the danger of basing thermodynamics on "hypotheses about the constitution of matter".[32] Gibbs's own framework for statistical mechanics was so carefully constructed that it could be carried over almost intact after the discovery that the microscopic laws of nature obey the rules of quantum mechanics, rather than the classical mechanics known to Gibbs and to his contemporaries.[6] His resolution of the so-called "Gibbs paradox," about the entropy of the mixing of gases, is now often cited as a prefiguration of the indistinguishability of particles required by quantum mechanics.[49]
Vector analysis
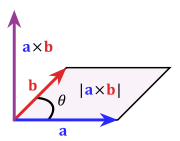
British scientists, including Maxwell, had relied on Hamilton's quaternions in order to express the dynamics of physical quantities, like the electric and magnetic fields, having both a magnitude and a direction in three-dimensional space. Gibbs, however, noted that the product of quaternions always had to be separated into two parts: a one-dimensional (scalar) quantity and a three-dimensional vector, so that the use of quaternions introduced mathematical complications and redundancies that could be avoided in the interest of simplicity and to facilitate teaching. He therefore proposed defining distinct dot and cross products for pairs of vectors and introduced the now common notation for them. He was also largely responsible for the development of the vector calculus techniques still used today in electrodynamics and fluid mechanics. In this effort, Gibbs was guided by the earlier work of Grassmann on "exterior algebra".[50]
As Gibbs had advocated in the 1880s and 1890s, quaternions were eventually all but abandoned by physicists in favor of the vectorial approach developed by him and, independently, by Oliver Heaviside. Gibbs applied his vector methods to the determination of planetary and comet orbits. He also developed the concept of mutually reciprocal triads of vectors that later proved to be of importance in crystallography.[51]
Physical optics
Though Gibbs's research on physical optics is less well known today than his other work, it made a significant contribution to classical electromagnetism by applying Maxwell's equations to the theory of optical processes such as birefringence, dispersion, and optical activity.[5][30] In that work, Gibbs showed that those processes could be accounted for by Maxwell's equations without any special assumptions about the microscopic structure of matter or about the nature of the medium in which electromagnetic waves were supposed to propagate (the so-called luminiferous aether). Gibbs also stressed that the absence of a longitudinal electromagnetic wave, which is needed to account for the observed properties of light, is automatically guaranteed by Maxwell's equations (by virtue of what is now called their "gauge invariance"), whereas in mechanical theories of light, such as Lord Kelvin's, it must be imposed as an ad hoc condition on the properties of the aether.[30]
Scientific recognition
Gibbs worked at a time when there was little tradition of rigorous theoretical science in the United States. His research was not easily understandable to his students or his colleagues and he made no effort to popularize his ideas or to simplify their exposition to make them more accessible.[6] His seminal work on thermodynamics was published mostly in the Transactions of the Connecticut Academy, a journal edited by his librarian brother-in-law, which was little read in the USA and even less so in Europe. When Gibbs submitted his long paper on the equilibrium of heterogeneous substances to the Academy, both Elias Loomis and Hubert Anson Newton protested that they did not understand Gibbs's work at all, but they helped to raise the money needed to pay for the typesetting of the many equations and mathematical symbols in the paper. Funds for the purpose were contributed both by members of the university and by local business and professional men in New Haven.[52]
According to James Gerald Crowther,
in his later years [Gibbs] was a tall, dignified gentleman, with a healthy stride and ruddy complexion, performing his share of household chores, approachable and kind (if unintelligible) to students. Gibbs was highly esteemed by his friends, but American science was too preoccupied with practical questions to make much use of his profound theoretical work during his lifetime. He lived out his quiet life at Yale, deeply admired by a few able students but making no immediate impress on American science commensurate with his genius.
— J. G. Crowther, 1937[6]
In his autobiography, mathematician Gian-Carlo Rota tells of casually browsing the mathematical stacks of Sterling Library and stumbling on a handwritten mailing list, attached to some of Gibbs's course notes, which listed over two hundred notable scientists of his day, including Poincaré, Boltzmann, David Hilbert, and Ernst Mach.[53] (Lynde Wheeler reproduces this mailing list in an appendix to his biography of Gibbs.[54]) One may conclude that Gibbs's work was better known among the scientific elite of his day than the published material suggests.[53] Gibbs succeeded in interesting his European correspondents in that work, which was translated into German (then the leading language for chemistry) by Wilhelm Ostwald in 1892 and into French by Henri Louis Le Châtelier in 1899.
Gibbs received the major honors then possible for an academic scientist in the US: he was elected to the National Academy of Sciences in 1879 and was awarded the 1880 Rumford Prize from the American Academy of Arts and Sciences for his work on chemical thermodynamics.[55] He was also granted honorary doctorates from Princeton University and Williams College in the US, and from the universities of Erlangen and Christiania (now Oslo) in Europe.[5] He was inducted as a foreign member of the Royal Society of London in 1897 and received the Society's Copley Medal in 1901.[6] At the time, that was considered the highest international honor in the natural sciences.[1] Gibbs was also a corresponding member of the Prussian and French Academies of Science.[5]
Influence
Gibbs's most immediate and obvious influence was on physical chemistry and statistical mechanics, two disciplines which he greatly helped to found. During Gibbs's lifetime, his phase rule was experimentally validated by Dutch chemist H. W. Bakhuis Roozeboom, who showed how to apply it in a variety of situations, thereby assuring it of widespread use.[56] In industrial chemistry, Gibbs's thermodynamics found many applications in the early 20th century, from electrochemistry to the development of the Haber process for the synthesis of ammonia.[57]
When Dutch physicist J. D. van der Waals received the 1910 Nobel Prize "for his work on the equation of state for gases and liquids" he acknowledged the great influence of Gibbs's work on that subject.[58] Max Planck received the 1918 Nobel Prize for his work on quantum mechanics, particularly his 1900 paper on the quantization of black-body radiation (see Planck's law). That work was based largely on the thermodynamics of Kirchhoff, Boltzmann, and Gibbs. According to Planck, Gibbs's name "not only in America but in the whole world will ever be reckoned among the most renowned theoretical physicists of all times."[59]

The first half of the 20th century saw the publication of two influential textbooks that soon came to be regarded as founding documents of chemical thermodynamics, both of which used and extended Gibbs's work in that field: these were Thermodynamics and the Free Energy of Chemical Processes (1923), by Gilbert N. Lewis and Merle Randall, and Modern Thermodynamics by the Methods of Willard Gibbs (1933), by Edward A. Guggenheim.[60] Under the influence of Lewis, William Giauque (who had originally wanted to be a chemical engineer) went on to become a professor of chemistry at Berkeley and won the 1949 Nobel Prize in Chemistry for his investigations into the properties of matter at temperatures close to absolute zero, studies guided by the third law of thermodynamics.[61]
Gibbs's early papers on the use of graphical methods in thermodynamics reflect a powerfully original understanding of what mathematicians would later call convex analysis, including ideas that, according to Barry Simon, "lay dormant for about seventy-five years".[62] Gibbs's later work on statistical ensembles, as presented in his 1902 textbook, has had an even greater impact in both theoretical physics and in pure mathematics.[47][48] Initially unaware of Gibbs's contributions in that field, Albert Einstein wrote three papers on statistical mechanics, published between 1902 and 1904. After reading Gibbs's textbook (which was translated into German by Ernst Zermelo in 1905), Einstein declared that Gibbs's treatment was superior to his own and explained that he would not have written those papers if he had known Gibbs's work.[63] According to mathematical physicist Arthur Wightman:
It is one of the striking features of the work of Gibbs, noticed by every student of thermodynamics and statistical mechanics, that his formulations of physical concepts were so felicitously chosen that they have survived 100 years of turbulent development in theoretical physics and mathematics.
— A. S. Wightman, 1990[48]
The development of vector calculus was Gibbs's other great contribution to mathematics. The publication in 1901 of E. B. Wilson's textbook Vector Analysis, based on Gibbs's lectures at Yale, did much to propagate the use of vectorial methods and notation in both mathematics and theoretical physics, definitively displacing the quaternions that had until then been dominant in the scientific literature.[64]
At Yale, Gibbs was also the mentor of Lee De Forest, who went on to invent to the triode amplifier and has been called the "father of radio".[65] According to De Forest, it was thanks to Gibbs that he realized early on "that the leaders in electrical development would be those who pursued the higher theory of waves and oscillations and the transmission by these means of intelligence and power."[36] Another student of Gibbs who played a significant role in the development of radio technology was Lynde Wheeler.
Gibbs also had an indirect influence on mathematical economics. He supervised the thesis of Irving Fisher, who received the first Ph.D. in economics from Yale in 1891. In that work, published in 1892 as Mathematical Investigations in the Theory of Value and Prices, Fisher drew a direct analogy between Gibbsian equilibrium in physical and chemical systems, and the general equilibrium of markets, and he used Gibbs's vectorial notation.[34][66] Gibbs's protegé Edwin Bidwell Wilson became, in turn, a mentor to leading American economist and Nobel Laureate Paul Samuelson.[67] In 1947, Samuelson published Foundations of Economic Analysis, based on his doctoral dissertation, in which he used as epigraph a remark attributed to Gibbs: "Mathematics is a language." Samuelson explicitly acknowledged the influence of Gibbs's classical thermodynamic methods[67] and identified him as "Yale's great physicist".[68]
For his part, mathematician Norbert Wiener cited Gibbs's use of probability in the formulation of statistical mechanics as "the first great revolution of twentieth century physics" and as a major influence on his conception of cybernetics. Wiener explained in the preface to his book The Human Use of Human Beings that it was "devoted to the impact of the Gibbsian point of view on modern life, both through the substantive changes it has made to working science, and through the changes it has made indirectly in our attitude to life in general."[69]
Commemoration

When the German physical chemist Walther Nernst visited Yale in 1906 to give the Silliman lecture, he was surprised to discover that there was no tangible memorial for Gibbs. He therefore donated his $500 lecture fee to the university to help pay for a suitable monument, which was finally unveiled in 1912 in the form of a bronze bas-relief by sculptor Lee Lawrie, installed in the Sloane Physics Laboratory.[70] In 1910, the American Chemical Society established the Willard Gibbs Medal, through the initiative of William A. Converse, a former chairman and secretary of the Chicago Section.[71] The American Mathematical Society endowed the Josiah Willard Gibbs Lectureship in 1923 to increase public awareness of mathematics and its applications.[72]
In 1945, Yale University created the J. Willard Gibbs Professorship in Theoretical Chemistry, held until 1973 by Lars Onsager, who won the 1968 Nobel Prize in chemistry. (Onsager, like Gibbs, worked primarily on the application of new mathematical ideas to problems in physical chemistry.) Yale's Josiah Willard Gibbs Laboratories and its J. Willard Gibbs Assistant Professorship in Mathematics are also named in his honor, and the university has hosted two symposia dedicated to Gibbs's life and work, one in 1989 and another on the centenary of his death, in 2003.[73] Rutgers University has a J. Willard Gibbs Professorship of Thermomechanics, presently held by Bernard D. Coleman.[74]
In 1950, Gibbs was elected to the Hall of Fame for Great Americans.[75] The United States Navy oceanographic research ship USNS Josiah Willard Gibbs (T-AGOR-1), in service from 1958 to 1971, was named for Gibbs.[76]
E. A. Guggenheim introduced the symbol G for the Gibbs free energy in 1933, and the same symbol was used also by Dirk ter Haar in 1966.[77] This notation is now universal and is recommended by the IUPAC.[78] In 1960, William Giauque et al. suggested using the name "gibbs" (abbreviated gbs.) for the unit of entropy, calorie / Kelvin,[79] but this usage did not become common and the corresponding SI unit, Joule / Kelvin, carries no special name.
Gibbs stamp (2005)

On May 4, 2005, the United States Postal Service issued the American Scientists commemorative postage stamp series designed by artist Victor Stabin, depicting Gibbs, John von Neumann, Barbara McClintock, and Richard Feynman.[80] Kenneth R. Jolls, a professor of chemical engineering at Iowa State University and an expert on graphical methods in thermodynamics, consulted on the design of the stamp honoring Gibbs.[81][82][83] The stamp identifies Gibbs as a "thermodynamicist" and features a diagram from the 4th edition of Maxwell's Theory of Heat, published in 1875, which illustrates Gibbs's thermodynamic surface for water.[82][83] Microprinting on the collar of Gibbs's portrait depicts his original mathematical equation for the change in the energy of a substance in terms of its entropy and the other state variables.[84]
In literature
In 1909, the American historian and novelist Henry Adams finished an essay entitled "The Rule of Phase Applied to History", in which he sought to apply Gibbs's phase rule and other thermodynamic concepts to a general theory of human history. William James, Henry Bumstead, and others criticized both Adams's tenuous grasp of the scientific concepts that he invoked, as well as the arbitrariness of his application of those concepts as metaphors for the evolution of human thought and society.[85] The essay remained unpublished until it appeared posthumously in 1919, in The Degradation of the Democratic Dogma, edited by Henry Adams's younger brother Brooks.[86]
In the 1930s, feminist poet Muriel Rukeyser became fascinated by Willard Gibbs and wrote a long poem about his life and work ("Gibbs", included in the collection A Turning Wind, published in 1939), as well as a book-length biography (Willard Gibbs, 1942).[87] According to Rukeyser:
Willard Gibbs is the type of the imagination at work in the world. His story is that of an opening up which has had its effect on our lives and our thinking; and, it seems to me, it is the emblem of the naked imagination—which is called abstract and impractical, but whose discoveries can be used by anyone who is interested, in whatever "field"—an imagination which for me, more than that of any other figure in American thought, any poet, or political, or religious figure, stands for imagination at its essential points.
— Muriel Rukeyser, 1949[88]
Both Gibbs and Rukeyser's biography of him figure prominently in the poetry collection True North (1997) by Stephanie Strickland.[89]
Gibbs's nephew, Ralph Gibbs Van Name, a professor of physical chemistry at Yale, was unhappy with Rukeyser's biography, in part because of her lack of scientific training. Van Name had withheld the family papers from her and, after her book was published in 1942 to positive literary but mixed scientific reviews, he tried to encourage Gibbs's former students to produce a new and more technically oriented biography.[90] With his support, Lynde Wheeler published such a work in 1951.[91]
In fiction, Gibbs appears as the mentor to character Kit Traverse in Thomas Pynchon's novel Against the Day (2006). That novel also prominently discusses the birefringence of Iceland spar, an optical phenomenon that Gibbs investigated.[92]
Outline of principal work
- Statistical mechanics: statistical ensemble, phase space, Gibbs entropy, ergodic hypothesis, Gibbs paradox
- Physical chemistry: Matter phase, chemical potential, Gibbs phase rule, enthalpy, free energy, phase diagram
- Electromagnetism: Maxwell's equations, birefringence
- Mathematics: Vector Analysis (Gibbs/Wilson), cross product, Gibbs phenomenon
See also
- Timeline of thermodynamics
- List of notable textbooks in statistical mechanics
- List of theoretical physicists
References
- ^ a b c "J. Willard Gibbs". Physics History. American Physical Society. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Copley Medal". Premier Awards. Royal Society. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Millikan, Robert A. (1938). "Biographical Memoir of Albert Abraham Michelson, 1852–1931" (PDF). Biographical Memoirs of the National Academy of Sciences of the United States of America. 19 (4): 121–146.
- ^ Linder, Douglas. "Biography of Prof. Josiah Gibbs". Famous American Trials: Amistad Trial. University of Missouri-Kansas City School of Law. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b c d e f g h i j k Bumstead 1928
- ^ a b c d e f g O'Connor, John J.; Robertson, Edmund F. (1997). "Josiah Willard Gibbs". The MacTutor History of Mathematics archive. University of St Andrews, Scotland. School of Mathematics and Statistics. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b Rukeyser 1988, p. 104
- ^ Rukeyser 1988, p. 430
- ^ a b Wheeler 1998, pp. 23–4
- ^ Rukeyser 1998, pp. 120, 142
- ^ Wheeler 1998, p. 30
- ^ Rukeyser 1998, p. 134
- ^ a b Wheeler 1998, p. 32
- ^ US Patent No. 53,971, "Car Brake", Apr. 17, 1866. See The Early Work of Willard Gibbs in Applied Mechanics, (New York: Henry Schuman, 1947), pp. 51–62.
- ^ Wheeler 1998, appendix II
- ^ Wheeler 1998, p. 44
- ^ Wheeler 1998, ch. III
- ^ Klein, Martin J. (1990). "The Physics of J. Willard Gibbs in His Time". Proceedings of the Gibbs Symposium. pp. 1–22.
- ^ Attention: This template ({{cite jstor}}) is deprecated. To cite the publication identified by jstor:531164, please use {{cite journal}} with
|jstor=531164instead. - ^ Wheeler 1998, pp. 54–5
- ^ Rukeyser 1988, pp. 181–2
- ^ Wheeler 1998, pp. 57–9
- ^ Kriz, Ronald D. (2007). "Thermodynamic Case Study: Gibbs' Thermodynamic Graphical Method". Virginia Tech, Dept. of Engineering Science and Mechanics. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Rukeyser 1988, p. 201
- ^ Rukeyser 1988, p. 251
- ^ Quoted in Rukeyser 1988, p. 233
- ^ a b c Wheeler 1998, ch. V
- ^ Wheeler 1998, p. 91
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF00330404, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF00330404instead. - ^ a b c Wheeler 1998, ch. VIII
- ^ a b c d e f Klein 2008
- ^ a b c Wheeler 1998, ch. X
- ^ a b c Wilson 1931
- ^ a b Fisher, Irving (1930). "The application of mathematics to the social sciences" (PDF). Bulletin of the American Mathematical Society. 36 (4): 225–243. doi:10.1090/S0002-9904-1930-04919-8.
- ^ Fisher, George W. (2005). "Foreword". Celebrating Irving Fisher: The Legacy of a Great Economist. Wiley-Blackwell.
- ^ a b Schiff, Judith Ann (Nov./Dec. 2008). "The man who invented radio". Yale Alumni Magazine. Retrieved 24 Jun. 2012.
{{cite news}}: Check date values in:|accessdate=and|date=(help) - ^ "Josiah Willard Gibbs". Find A Grave. Retrieved 19 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b Wheeler 1998, pp. 179–180
- ^ a b c d Seeger 1974, pp. 15–6
- ^ Obituary Record of Graduates of Yale University, 1901–1910. New Haven: Tuttle, Morehouse & Taylor. 1910. p. 238.
- ^ a b Wheeler, 1998, p. 16
- ^ Samuelson, Paul A. (1990). "Gibbs in Economics". Proceedings of the Gibbs Symposium. p. 255.
- ^ Rukeyser 1988, pp. 254, 345, 430
- ^ Wheeler 1998, p. 95. See also the Collected Works, vol. II
- ^ Wheeler, 1998, p. 144
- ^ Poincaré, Henri (1904), , The Foundations of Science (The Value of Science), New York: Science Press, pp. 297–320
- ^ a b Wiener, Norbert (1961). "II: Groups and Statistical Mechanics". Cybernetics: or Control and Communication in the Animal and the Machine (2 ed.). MIT Press. ISBN 978-0-262-23007-0.
- ^ a b c Wightman, Arthur S. (1990). "On the Prescience of J. Willard Gibbs". Proceedings of the Gibbs Symposium. pp. 23–38.
- ^ See, e.g., Huang, Kerson (1987). Statistical Mechanics (2 ed.). John Wiley & Sons. pp. 140–143. ISBN 0-471-81518-7.
- ^ Wheeler 1998, p. 109
- ^ Shmueli, Uri (2006). "Reciprocal Space in Crystallography". International Tables for Crystallography. Vol. B. pp. 2–9.
- ^ Rukeyser 1998, pp. 225–6
- ^ a b Rota, Gian-Carlo (1996). Indiscrete Thoughts. Birkhäuser. p. 25. ISBN 978-0-8176-3866-5.
- ^ Wheeler 1998, appendix IV
- ^ Müller, Ingo (2007). A History of Thermodynamics - the Doctrine of Energy and Entropy. Springer. ISBN 978-3-540-46226-2.
- ^ Crowther, James Gerald (1969 [1937]). "Josiah Willard Gibbs, 1839–1903". Famous American Men of Science. Freeport, NY: Books for Libraries. pp. 277–8.
{{cite book}}: Check date values in:|year=(help)CS1 maint: year (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0016-0032(25)90344-4, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0016-0032(25)90344-4instead. - ^ van der Waals, J. D. (1910). "Nobel Lecture: The Equation of State for Gases and Liquids". Nobel Prize in Physics. Nobel Foundation.
- ^ Planck, Max (1915). "Second Lecture: Thermodynamic States of Equilibrium in Dilute Solutions". Eight Lectures on Theoretical Physics. New York: Columbia University Press. p. 21.
- ^ Ott, Bevan J. (2000). Chemical Thermodynamics – Principles and Applications. Academic Press. ISBN 0-12-530990-2.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tiselius, Arne (1949). "Award Ceremony Speech". Nobel Prize in Chemistry. Nobel Foundation.
- ^ Simon, Barry (2011). Convexity: An Analytic Viewpoint. Cambridge University Press. p. 287. ISBN 1-1070-0731-3.
- ^ Marsden, Jerrold E.; Tromba, Anthony J. (1988). Vector Calculus (3 ed.). W. H. Freeman. p. 18. ISBN 0-7167-1856-1.
- ^ Seeger 1974, p. 18
- ^ Leontief, Wassily (1954). "Mathematics in economics". Bulletin of the American Mathematical Society. 60 (3): 215–233. doi:10.1090/S0002-9904-1954-09791-4.
- ^ a b Samuelson, Paul A. (1970). "Maximum Principles in Analytical Economics" (PDF). Nobel Prize Lecture. Nobel Foundation.
- ^ Samuelson, Paul A. (5 Sep. 2003). "How I Became an Economist". Prize in Economic Sciences. Nobel Foundation. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=and|date=(help) - ^ Wiener, Norbert (1950). The Human Use of Human Beings: Cybernetics and Society. Houghton Mifflin. pp. 10–11.
- ^ Seeger 1974, p. 21
- ^ "The Willard Gibbs Medal Founded by William A. Converse". American Chemical Society, Chicago Section. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Josiah Willard Gibbs Lectures". Special Lectures. American Mathematical Society. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "Forum News" (PDF). History of Physics Newsletter. 8 (6): 3. 2003.
- ^ Coleman, Bernard D. "Faculty webpage". Rutgers University, Dept. of Mechanics and Materials Science. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Johnson, D. Wayne. "The Hall of Fame for Great Americans at New York University". Medal Collectors of America. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ "San Carlos". Dictionary of American Naval Fighting Ships. Naval History and Heritage Command. Retrieved 16 Jun. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Seeger 1974, p. 96
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1351/goldbook.G02629, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1351/goldbook.G02629instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01486a014, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01486a014instead. - ^ "Yale scientist featured in new stamp series". Yale Bulletin & Calendar. Vol. 33, no. 28. 20 May 2005. Retrieved 30 Nov. 2012.
{{cite news}}: Check date values in:|accessdate=(help) - ^ "Iowa State Chemical Engineer Drives Issue of New Stamp Honoring Father of Thermodynamics". College Feature, Iowa State University, College of Engineering. 2004. Retrieved 17 Nov. 2012.
{{cite news}}: Check date values in:|accessdate=(help) - ^ a b Hacker, Annette (11 Nov. 2004). "ISU professor helps develop postage stamp honoring noted scientist". News Service, Iowa State University. Retrieved 17 Nov. 2012.
{{cite news}}: Check date values in:|accessdate=and|date=(help) - ^ a b "Postal Service Pays Homage to Josiah Willard Gibbs". Chemical Engineering Progress. 101 (7): 57. 2005.
- ^ Spakovszky, Zoltan (2005). "Stamp of Authenticity" (PDF). ASME Mechanical Engineering. 128 (4): 7.
- ^ Attention: This template ({{cite jstor}}) is deprecated. To cite the publication identified by jstor:2708401, please use {{cite journal}} with
|jstor=2708401instead. - ^ Adams, Henry (1919). The Degradation of the Democratic Dogma. New York: Macmillan. Retrieved 5 May 2012.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rukeyser 1988
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1063/1.3066422, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1063/1.3066422instead. - ^ Strickland, Stephanie (1997). True North. Notre Dame, IN: University of Notre Dame Press. ISBN 978-0-268-01899-3.
- ^ Holeman, Heather L. (1986). "Guide to the Gibbs-Van Name Papers". Yale University Library. Retrieved 18 Nov. 2012.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Wheeler 1998
- ^ Pynchon, Thomas (2006). Against the Day. New York: Penguin. ISBN 978-1-59420-120-2.
Bibliography
Primary
- The Early Work of Willard Gibbs in Applied Mechanics, eds. L. P. Wheeler, E. O. Waters and S. W. Dudley, (New York: Henry Schuman, 1947). ISBN 1-881987-17-5
- J. W. Gibbs, "On the Equilibrium of Heterogeneous Substances", Transactions of the Connecticut Academy of Arts and Sciences, 3, 108–248, 343–524, (1874–1878). Reproduced in both the Scientific Papers (1906) and the Collected Works of J. Willard Gibbs (1928).
- E. B. Wilson, Vector Analysis, a text-book for the use of students of Mathematics and Physics, founded upon the Lectures of J. Willard Gibbs, (New Haven: Yale University Press, 1929 [1901]).
- J. W. Gibbs, Elementary Principles in Statistical Mechanics, developed with especial reference to the rational foundation of thermodynamics, (New York: Dover Publications, 1960 [1902]).
- The Scientific Papers of J. Willard Gibbs, in two volumes, eds. H. A. Bumstead and R. G. Van Name, (Woodbridge, CT: Ox Bow Press, 1993 [1906]). ISBN 0-918024-77-3, ISBN 1-881987-06-X. For scans of the 1906 printing, see vol. I and vol. II.
- The Collected Works of J. Willard Gibbs, in two volumes, eds. W. R. Longley and R. G. Van Name, (New Haven: Yale University Press, 1957 [1928]).
Secondary
- References for J Willard Gibbs, The MacTutor History of Mathematics archive, University of St Andrews, School of Mathematics and Statistics (1997). Retrieved 3 Dec. 2012.
- H. A. Bumstead, "Josiah Willard Gibbs", American Journal of Science, 16, 187–202 (1903), reprinted with some additions in both the Scientific Papers (1906) and the Collected Works of J. Willard Gibbs (1928), pp. xiii–xxviiii.
- Proceedings of the Gibbs Symposium, Yale University, May 15–17, 1989, eds. D. G. Caldi and G. D. Mostow, (American Mathematical Society and American Institute of Physics, 1990).
- M. J. Crowe, A History of Vector Analysis: The Evolution of the Idea of a Vectorial System, (New York: Dover, 1994 [1967]). ISBN 0-486-67910-1
- J. G. Crowther, Famous American Men of Science, (Freeport, NY: Books for Libraries Press, 1969 [1937]). ISBN 0-8369-0040-5
- A Commentary on the Scientific Writings of J. Willard Gibbs, in two volumes, eds. F. G. Donnan and A. E. Hass, (New York: Arno, 1980 [1936]). ISBN 0-405-12544-5
- P. Duhem, Josiah-Willard Gibbs à propos de la publication de ses Mémoires scientifiques, (Paris: A. Herman, 1908).
- C. S. Hastings, "Josiah Willard Gibbs", Biographical Memoirs of the National Academy of Sciences, 6, 373–393 (1909).
- M. J. Klein, "Gibbs, Josiah Willard", in Complete Dictionary of Scientific Biography, vol. 5, (Detroit: Charles Scriber's Sons, 2008), pp. 386–93.
- K. Meinke and J. V. Tucker, "Universal Algebra", in Handbook of Logic in Computer Science, vol. I, eds. S. Abramsky, D. Gabbay and T. S. E. Maibaum, (Oxford: Oxford University Press), pp. 189–411. ISBN 0-19-853761-1
- M. Rukeyser, Willard Gibbs: American Genius, (Woodbridge, CT: Ox Bow Press, 1988 [1942]). ISBN 0-918024-57-9
- R. J. Seeger, J. Willard Gibbs, American mathematical physicist par excellence, (Oxford and New York: Pergamon Press, 1974). ISBN 0-08-018013-2
- L. P. Wheeler, Josiah Willard Gibbs, The History of a Great Mind, (Woodbridge, CT: Ox Bow Press, 1998 [1951]). ISBN 1-881987-11-6
- E. B. Wilson, "Reminiscences of Gibbs by a student and colleague", Bulletin of the American Mathematical Society, 37, 401–416 (1931).
External links
- O'Connor, John J.; Robertson, Edmund F., "Josiah Willard Gibbs", MacTutor History of Mathematics Archive, University of St Andrews
- "Josiah Willard Gibbs", American Institute of Physics (2003 [1976]).
- Josiah Willard Gibbs at the Mathematics Genealogy Project
- 1839 births
- 1903 deaths
- Thermodynamicists
- Physical chemists
- Mathematical analysts
- American chemists
- American physicists
- American mathematicians
- 19th-century mathematicians
- Foreign Members of the Royal Society
- Yale University alumni
- University of Heidelberg alumni
- Yale University faculty
- Hopkins School alumni
- People from New Haven, Connecticut
- Recipients of the Copley Medal
- Burials at Grove Street Cemetery
- Theoretical physicists


