Dementia with Lewy bodies: Difference between revisions
SandyGeorgia (talk | contribs) →Other: add Walker |
SandyGeorgia (talk | contribs) →Essential features: Walker |
||
| Line 43: | Line 43: | ||
===Essential features=== |
===Essential features=== |
||
[[Dementia]] is present, but does not always appear early on with DLB. It is more likely to appear as the condition progresses,<ref name=McKeithConsensus2017/> typically after age 55.<ref name=Gomperts2016/> In contrast to [[Alzheimer's disease]] (AD), in which [[episodic memory]] loss related to encoding of memories is typically the earliest symptom,<ref name=Karant2011>{{cite journal |vauthors=Karantzoulis S, Galvin JE |title=Distinguishing Alzheimer's disease from other major forms of dementia |journal=Expert Rev Neurother |volume=11 |issue=11 |pages=1579–91 |date=November 2011 |pmid=22014137 |pmc=3225285 |doi=10.1586/ern.11.155 |type=Review}}</ref> patients with LBD have better verbal memory, memory is affected later in the progression of the disease, and memory problems are related to retrieval of memories rather than encoding of new memories.<ref name=Gomperts2016/><ref name=Karant2011/> Those with DLB experience impaired attention, [[executive function]], and visuospatial function, that present as driving difficulty, such as becoming lost, misjudging distances, or as impaired job performance, difficulty multitasking, or inability to follow conversations.<ref name=Gomperts2016/> Difficulties with visuospatial processing are present in most individuals with DLB,<ref name=Walker2015/> and they show up earlier and are more pronounced than in AD.<ref name=Karant2011/> Dementia is diagnosed when the "progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational functions, or with usual daily activities".<ref name=McKeithConsensus2017/> |
[[Dementia]] is present, but does not always appear early on with DLB. It is more likely to appear as the condition progresses,<ref name=McKeithConsensus2017/> typically after age 55.<ref name=Gomperts2016/> In contrast to [[Alzheimer's disease]] (AD), in which [[episodic memory]] loss related to encoding of memories is typically the earliest symptom,<ref name=Karant2011>{{cite journal |vauthors=Karantzoulis S, Galvin JE |title=Distinguishing Alzheimer's disease from other major forms of dementia |journal=Expert Rev Neurother |volume=11 |issue=11 |pages=1579–91 |date=November 2011 |pmid=22014137 |pmc=3225285 |doi=10.1586/ern.11.155 |type=Review}}</ref> patients with LBD have better verbal memory, memory is affected later in the progression of the disease, and memory problems are related to retrieval of memories rather than encoding of new memories.<ref name=Gomperts2016/><ref name=Karant2011/> Those with DLB experience impaired attention, [[executive function]], and visuospatial function, that present as driving difficulty, such as becoming lost, misjudging distances, or as impaired job performance, difficulty multitasking, or inability to follow conversations.<ref name=Gomperts2016/> Difficulties with visuospatial processing are present in most individuals with DLB,<ref name=Walker2015/> and they show up earlier and are more pronounced than in AD.<ref name=Karant2011/> While 74% of patients with early DLB confirmed at autopsy have these deficits, they show up in only 45% of patients with AD.<ref name=Walker2015/> Dementia is diagnosed when the "progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational functions, or with usual daily activities".<ref name=McKeithConsensus2017/> |
||
===Core features=== |
===Core features=== |
||
Revision as of 18:04, 24 March 2018
| Dementia with Lewy bodies | |
|---|---|
| Other names | Diffuse Lewy body disease, cortical Lewy body disease, senile dementia of Lewy type |
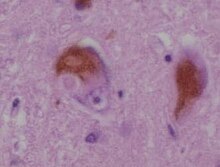 | |
| A microscopic image of Lewy bodies | |
| Specialty | Neurology |
| Symptoms | Dementia, abnormal behavior during REM sleep, fluctuations in alertness, visual hallucinations, slowness of movement[1] |
| Usual onset | After the age of 50[2] |
| Duration | Long term[3] |
| Causes | Unknown[3] |
| Diagnostic method | Based on symptoms after ruling out other conditions[failed verification][4] |
| Differential diagnosis | Parkinson's disease dementia, Alzheimer's disease[3] |
| Medication | Acetylcholinesterase inhibitors such as donepezil[3] |
| Frequency | 1 in 1,000 per year[5] |
Dementia with Lewy bodies (DLB) is a type of dementia that worsens over time, along with changes in behavior, cognition, and movement.[2] Symptoms may include fluctuations in alertness, abnormal behavior during REM sleep, visual hallucinations, slowness of movement, trouble walking, and rigidity.[1] Movements and abnormal behaviors during sleep are a core feature of DLB, called REM sleep behavior disorder.[1][6] Urinary incontinence and mood changes such as depression are also common.[7] DLB is classified as a neurodegenerative disorder.[3] Together with Parkinson's disease dementia (PDD), it is one of two dementias referred to as the Lewy body dementias (LBD).[8][9]
The exact cause is unknown.[3] Typically, no family history of the disease exists among those affected.[3] The underlying mechanism involves the buildup of Lewy bodies, clumps of alpha-synuclein protein in neurons.[3] A diagnosis may be suspected based on symptoms, with blood tests and medical imaging done to rule out other possible causes of the symptoms.[needs update][dubious – discuss][4] The differential diagnosis includes Parkinson's disease dementia and Alzheimer's disease (AD).[3]
At present there is no cure.[3] Treatments are supportive and attempt to relieve some of the symptoms associated with the disease.[3] Acetylcholinesterase inhibitors, such as donepezil, may provide some benefit,[3] and melatonin can be used for sleep symptoms.[1] Antipsychotics, even for hallucinations, should generally be avoided due to sensitivity of people with DLB to these medications.[1][3] Such use can potentially result in death.[10]
DLB is the third most common cause of dementia after Alzheimer's disease and vascular dementia.[2] It typically begins after the age of 50.[2] About 1 in 1,000 people are newly affected each year.[5] Men appear to be more commonly affected than women.[11] In the late part of the disease, people may depend entirely on others for their care.[2] Life expectancy following diagnosis is about eight years.[3] The abnormal deposits that cause the disease were discovered in 1912 by Frederic Lewy.[12]
Classification
Dementia with Lewy bodies is a progressive neurogenerative dementia;[13] together with Parkinson's disease dementia, it is one of the Lewy body dementias.[8] Along with Parkinson's disease, multiple system atrophy, and other more rare conditions, they make up the synucleinopathies—neurodegerative diseases that are due to an abnormal accumulation of alpha-synuclein protein in the brain.[14]
After Alzheimer's disease, LBD is the next most common dementia.[11]
Signs and symptoms
The symptoms of DLB can be divided into essential, core and supportive features.[13]
Essential features
Dementia is present, but does not always appear early on with DLB. It is more likely to appear as the condition progresses,[1] typically after age 55.[8] In contrast to Alzheimer's disease (AD), in which episodic memory loss related to encoding of memories is typically the earliest symptom,[15] patients with LBD have better verbal memory, memory is affected later in the progression of the disease, and memory problems are related to retrieval of memories rather than encoding of new memories.[8][15] Those with DLB experience impaired attention, executive function, and visuospatial function, that present as driving difficulty, such as becoming lost, misjudging distances, or as impaired job performance, difficulty multitasking, or inability to follow conversations.[8] Difficulties with visuospatial processing are present in most individuals with DLB,[11] and they show up earlier and are more pronounced than in AD.[15] While 74% of patients with early DLB confirmed at autopsy have these deficits, they show up in only 45% of patients with AD.[11] Dementia is diagnosed when the "progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational functions, or with usual daily activities".[1]
Core features
While the specific symptoms in a person with DLB may vary, core features designated by the 2017 DLB Consortium are:[1]
- fluctuating cognition, alertness or attention;
- REM sleep behavior disorder (RBD);
- "spontaneous cardinal features of parkinsonism"; and
- repeated visual hallucinations (VH).[1]
Core features are based on "their diagnostic specificity and the volume of good-quality evidence available",[1] although in other severe cases of dementia (for example, advanced stages of Alzheimer's), some core features of DLB may also be present.[11]
Fluctuating cognition, alertness or attention
In DLB, "marked attentional and executive function disturbance is central" and "attentional disturbance may serve as the basis of fluctuating cognition that is characteristic".[15] Individuals with DLB may be easily distracted, and have a hard time focusing on tasks,[15] or appear to be "delirium-like", "zoning out", or in states of altered consciousness.[1] They may also exhibit disorganized speech; daytime sleepiness, drowsiness or napping; and changing ability to organize their thoughts during the day. Attention, thinking and alertness fluctuates throughout the day, and is often present early in the course of the disease,[1][8] unlike in AD, where it is unclear if executive function is impacted early.[15]
REM sleep behavior disorder
This section needs expansion. You can help by adding to it. (March 2018) |
REM sleep behavior disorder (RBD) may appear decades before any other symptoms,[6] and often is a symptom first recognized by the patient's caretaker. RBD includes vivid dreaming, with persistent dreams, purposeful or violent movements, and falling out of bed.[16]
On autopsy, up to 98% of individuals with polysomnography-confirmed RBD are found to have a synucleinopathy.[17]
Parkinsonism
Parkinsonian features may include shuffling gait, reduced arm-swing during walking, blank expression (reduced range of facial expression), stiffness of movements, ratchet-like cogwheeling movements, low speech volume, sialorrhea, and difficulty swallowing.
Visual hallucinations
Up to 80% of people with DLB have visual hallucinations.[1] They commonly involve perception of people or animals that are not there, and may reflect Lewy bodies or AD pathology in the temporal lobe.[citation needed] These hallucinations are not necessarily disturbing, and in some cases, the person with DLB may have insight into the hallucinations and even be amused by them, or be conscious they are not real. People with DLB also may have problems with vision and misinterpretation of what they see, for example, mistaking a pile of socks for snakes or a clothes closet for the bathroom.[citation needed]
Supportive features
According to the DLB Consortium, "Although carrying less diagnostic weight, supportive items are often valuable in clinical decision-making, acting as signposts to or adding evidence for a DLB diagnosis."[1] Supportive features may persist over time, be present early in the progression of DLB, and are common, but they are not specific to the diagnosis.[1] The supportive features are:
- marked sensitivity to antipsychotics;
- marked dysautonomia (autonomic dysfunction);
- non-visual hallucinations;
- hypersomnia;
- hyposmia (reduced ability to smell);
- false beliefs and delusions organized around a common theme;
- "postural instability", loss of consciousness and frequent falls;
- "apathy, anxiety and depression".[1][13]
Antipsychotic sensitivity
It is no longer a core diagnostic feature, but severe sensitivity to antipsychotics is still cautioned for people with DLB[1] because half will have adverse reactions.[11] According to Boot (2015):
"The most fraught decision in the management of DLB relates to the use of antipsychotic medications ... DLB patients are particularly at risk of antipsychotic medication morbidity and mortality."[10]
Some medications that should be used with great caution, if at all, for people with DLB, are haloperidol,[10] chlorpromazine,[18] olanzapine,[19] risperidone,[19] thioridazine,[20] and injectable antipsychotics.[19]
Dysautonomia
Also, DLB patients often experience problems with orthostatic hypotension, including repeated falls, fainting, and transient loss of consciousness. Sleep-disordered breathing, a problem in multiple system atrophy, also may be a problem.[21]
Other
Psychiatric symptoms are more likely to cause more impairment, when compared to AD, and to be present when the individual first comes to clinical attention.[15] Symptoms that are more common in DLB than in the general population,[11] and may have been present for decades, anxiety affects 27% and depression affects 59% of people with DLB.[10] Agitation, behavioral disturbances,[10] and delusions may appear later in the course of the disease.[8] Delusions may have a paranoid quality, revolving around a house being broken in to or infidelity[8] As the individual with DLB misplaces items, they may have delusions centered around theft.[8] Capgras delusion may occur, where the person with DLB is convinced that an imposter has replaced their caregiver or spouse.[8]
Benzodiazepines, anticholinergics, surgical anesthetics, some antidepressants, and over-the-counter (OTC) cold remedies may cause acute confusion, delusions, and hallucinations.[citation needed]
Cause
The exact cause is not known, but DLB may have a genetic component; the DLB risk is heightened with inheritance of the ε4 allele of the apolipoprotein E (APOE).[13]
In DLB, loss of cholinergic (acetylcholine-producing) neurons is thought to account for degeneration of cognitive function (similar to Alzheimer's), while the death of dopaminergic (dopamine-producing) neurons appears to be responsible for degeneration of motor control (similar to Parkinson's)—in some ways, therefore, DLB resembles both disorders.
Pathophysiology

Pathologically, DLB is characterized by the development of abnormal collections of (alpha-synuclein) protein within the cytoplasm of neurons (known as Lewy bodies). These intracellular collections of protein have similar structural features to "classical" Lewy bodies, seen subcortically in Parkinson's disease. Additionally, those affected by DLB experience a loss of dopamine-producing neurons (in the substantia nigra) in a manner similar to that seen in Parkinson's disease.
A loss of acetylcholine-producing neurons (in the basal nucleus of Meynert and elsewhere) similar to that seen in Alzheimer's disease also is known to occur in those with DLB. Cerebral atrophy also occurs as the cerebral cortex degenerates. Autopsy series have revealed the pathology of DLB is often concomitant with the pathology of Alzheimer's disease. That is, when Lewy body inclusions are found in the cortex, they often co-occur with Alzheimer's disease pathology found primarily in the hippocampus, including senile plaques (deposited beta-amyloid protein), and granulovacuolar degeneration (grainy deposits within and a clear zone around hippocampal neurons). Neurofibrillary tangles (abnormally phosphorylated tau protein) are less common in DLB, although they are known to occur, and astrocyte abnormalities[vague] are also known to occur.[22][23]
Diagnosis
Dementia with Lewy bodies is often misdiagnosed[24] or confused in its early stages with Alzheimer's disease; in research settings, autopsy may reveal previously undiagnosed Lewy bodies in as many as half of Alzheimer's patients.[1] Despite the difficulty in diagnosis, a prompt diagnosis is important because of the serious risks of sensitivity to certain neuroleptic (antipsychotic) medications and the need to inform both the person with DLB and the person's caregivers about potentially irreversible side effects of those medications.[8]
The 2017 Fourth Consensus Report of the DLB Consortium established diagnostic criteria for probable and possible DLB, in recognition of advances in detection and improvements from the earlier (2005) version. The 2017 criteria are based on essential, core and supportive clinical features, and diagnostic biomarkers.
The essential feature is dementia, which must be sufficient to interfere with social or occupational functioning for a DLB diagnosis.[1] Dementia is diagnosed based on patient history, physical exam, and assessment of neurological function, and by ruling out conditions that may cause similar symptoms—conditions like depression, abnormal thyroid function, or vitamin deficiencies.[25]
The core clinical features (described in the Signs and symptoms section) are: fluctuating cognition, visual hallucinations, REM sleep behavior disorder, and signs of parkinsonism.[1]
The diagnostic biomarkers are:[1]
Indicative
- PET or SPECT showing reduced dopamine uptake in the basal ganglia;
- Abnormal iodine-MIBG myocardial scintigraphy;
- REM sleep without atonia evidenced on polysomnography; and
Supportive from PET, SPECT, CT, MRI or EEG brain studies showing:[1][13]
- preserved medial temporal lobe;
- low dopamine transporter uptake;
- reduced occipital activity; or
- prominent slow-wave activity.
Probable DLB can be diagnosed when dementia and at least two core features are present, or one core feature with at least one indicative biomarker is present.[1] Possible DLB can be diagnosed when dementia and only one core feature is present or if no core features are present, there is at least one indicative biomarker.[1]
Dementia with Lewy bodies is distinguished from Parkinson's disease dementia (PDD) by the time frame in which dementia symptoms appear relative to Parkinson symptoms.[1] PDD would be the diagnosis when dementia "occurs in the context of well-established Parkinson disease";[1] that is, typically (in a research setting) the onset of dementia is more than a year after the onset of Parkinsonian symptoms.[1] DLB is diagnosed when cognitive symptoms begin before or at the same time as parkinsonism.[1]
DLB is listed in the DSM-5 as "Major or Mild Neurocognitive Disorder with Lewy Bodies."[11] The differences between the two sets of criteria are:
- the DSM does not include low dopamine transporter uptake as a supportive feature,[11] and
- there is unclear diagnostic weight assigned to biomarkers in the DSM.[11]
Differential diagnosis
Alzheimer's, Parkinson's disease, and vascular dementia must be distinguished from DLB.[1] The Lewy body dementias (Parkinson disease dementia and dementia with Lewy bodies) are clinically similar after dementia occurs with Parkinson's disease.[11]
Management
No cure for dementia with Lewy bodies is known. Treatment may offer symptomatic benefit, but remains palliative in nature.[13] Management of DLB can be difficult because of the need to balance cognitive function, neuropsychiatric features and impairments related to the motor system.[8] Treatment modalities are divided into pharmaceutical and non-pharmaceutical.[8]
Medications
Pharmaceutical management, as with Parkinson's disease, involves striking a balance between treating the motor, emotive, and cognitive symptoms. Motor symptoms appear to respond somewhat to the medications used to treat Parkinson's disease (e.g. levodopa), while cognitive issues may improve with medications for Alzheimer's disease such as donepezil. Medications for both Parkinson's disease and ADHD increase levels of the chemical dopamine in the brain, so increase the risk of hallucinations with those classes of pharmaceuticals.[26]
Treatment of the movement and cognitive portions of the disease may worsen hallucinations and psychosis, while treatment of hallucinations and psychosis with antipsychotics may worsen parkinsonian or ADHD symptoms in DLB, such as tremor or rigidity and lack of concentration or impulse control.[27][28] There is strong evidence for the use of cholinesterase inhibitors as treatment for cognitive problems[29] and donepezil (Aricept), rivastigmine (Exelon), and galantamine (Reminyl) may be recommended as a means to help with these problems and to slow or prevent the decline of cognitive function.[citation needed] DLB may be more responsive to donepezil than Alzheimer's disease.[18] Memantine also may be useful.[30] Levocarb may help with movement problems, but in some cases, as with dopamine agonists, may tend to aggravate psychosis in people with DLB. Clonazepam may help with rapid eye movement behavior disorder; table salt or antihypotensive medications may help with fainting and other problems associated with orthostatic hypotension. Botulinum toxin injections in the parotid glands may help with sialorrhea.
To improve daytime alertness, there is mixed evidence for stimulants such as methylphenidate and dextromethamphetamine; they can increase the risk of psychosis, although worsening of neuropsychiatric symptoms is not common.[10][19] Modafinil and armodafinil are not always covered by insurance, but may be effective for daytime sleepiness.[19][10] Extreme caution in the use of antipsychotic medication in people with DLB because of their sensitivity to these agents. When these medications must be used, atypical antipsychotics are preferred to typical antipsychotics; a very low dose should be tried initially and increased slowly, and patients should be carefully monitored for adverse reactions to the medications.
Due to hypersensitivity to neuroleptics, preventing DLB patients from taking these medications is important. People with DLB are at risk for neuroleptic malignant syndrome, a life-threatening illness, because of their sensitivity to these medications, especially the older typical antipsychotics, such as haloperidol. Other medications, including medications for urinary incontinence and the antihistamine medication diphenhydramine (Benadryl), also may worsen confusion.
REM sleep behavior disorder may be treated with melatonin or clonazepam.[6]
Non-pharmaceutical and caregiving strategies
Because of the neuropsychiatric symptoms associated with DLB, the caregiver burden is higher than in Alzheimer's.[31] DLB gradually renders people incapable of tending to their own needs, so caregiving is important and must be managed carefully over the course of the disease. Caring for people with DLB involves adapting the home environment, schedule, activities, and communications to accommodate declining cognitive skills and parkinsonian symptoms.[32]
People with DLB may swing dramatically between good days, with high alertness and few cognitive or movement problems, and bad days, and the level of care they require thus may vary widely and unpredictably. Sharp changes in behavior may be due to the day-to-day variability of DLB, but they also may be triggered by changes in the schedule or home environment, or by physical problems, such as constipation, dehydration, bladder infection, injuries from falls, and other problems they may not be able to convey to caregivers. Potential physical problems always should be taken into consideration when an individual with DLB becomes agitated.
Visual hallucinations associated with DLB create a particular burden on caregivers.[33] As hallucinations and delusions are not necessarily dangerous or troubling to the person with DLB, caregivers not disabusing patients of them may be best. Often, the best approach is benign neglect—acknowledging, but not encouraging or agreeing. Trying to talk the DLB patient out of his delusion may be frustrating to caregivers and discouraging to patients, sometimes provoking anger or dejection. When misperceptions, hallucinations, and the behaviors stemming from these become troublesome, caregivers should try to identify and eliminate environmental triggers, and perhaps, offer cues or "therapeutic white lies" to steer patients out of trouble. Physicians may prescribe low doses of atypical antipsychotics, such as quetiapine, for psychosis and agitation in DLB. A small clinical trial found that about half of DLB patients treated with low doses of quetiapine experienced a significant reduction in these symptoms. Unfortunately, several participants in the study had to discontinue treatment because of side effects, such as excessive daytime sleepiness or orthostatic hypotension.[34]
Changes in the schedule or environment, delusions, hallucinations, misperceptions, and sleep problems also may trigger behavior changes. It can help people with DLB to encourage exercise, simplify the visual environment, stick to a routine, and avoid asking too much (or too little) of them. Speaking slowly and sticking to essential information improves communication. The potential for visual misperception and hallucinations, in addition to the risk of abrupt and dramatic swings in cognition and motor impairment, should put families on alert to the dangers of driving with DLB.[35]
Prognosis
Individuals with DLB "might have a less favourable prognosis, with accelerated cognitive decline, shorter lifespan, and increased admission to residential care" relative to individuals with Alzheimer's.[31]
Epidemiology
About 1 per 1,000 people newly develop the condition each year.[5] An estimated 10 to 15% of diagnosed dementias are Lewy body type, but estimates range as high as 24%.[11] Dementia with Lewy bodies tends to be under-recognized.[11] Dementia with Lewy bodies is slightly more common in men than women.[11] DLB increases in prevalence with age; the mean age at presentation is 75 years,[dubious – discuss][citation needed] although it is not uncommon for DLB to be diagnosed before the age of 65.[11]
Dementia with Lewy bodies affects about one million individuals in the United States.[citation needed]
History
Frederic Lewy (1885–1950) was the first to discover the abnormal protein deposits ("Lewy body inclusions") in the early 1900s.[8][12] Dementia with Lewy bodies was first described by Japanese psychiatrist and neuropathologist Kenji Kosaka in 1976.[13][36][37] DLB became easier to diagnose in the 1980s after the discovery of alpha-synuclein staining first highlighted Lewy bodies[8] in the cortex of post mortem brains of dementia patients.
DLB was included in the DSM-IV-TR (published in 2000) under "Dementia Due to Other General Medical Conditions."
Society and culture
Robin Williams, the American actor and comedian, died by suicide on August 11, 2014.[38] Upon autopsy, he was found to have diffuse DLB. Williams had been diagnosed with Parkinson's disease prior to his death; according to his wife, he also had depression, anxiety, and increasing paranoia.[39] Dementia researcher and professor of Old Age Psychiatry at Newcastle University, Ian McKeith, commented that DLB was too little known, and that Williams' symptoms were explained by DLB.[40]
The British author and poet Mervyn Peake died in 1968 and was diagnosed posthumously as a probable case of DLB in a 2003 study published in JAMA Neurology.[41] Although his death was "variously ascribed to Alzheimer disease, Parkinson disease, or postencephalitic parkinsonism", based on signs of progressive deterioration, fluctuating cognitive decline, deterioration in visuospatial function, declining attention span, and records in his work and letters of visual hallucinations and delusions, he may be the earliest known case of DLB.[41] Also in the world of publishing, Otis Chandler, of the Los Angeles Times, died from the disease in 2006.[42]
Among people in the performing arts, Estelle Getty, an actress known for her role in the television series The Golden Girls, suffered from DLB in her later years.[43] Another actress, socialite, and heiress Dina Merrill died in 2017 from DLB.[44] In 2015, the artist who created the plastic pink flamingo, Donald Featherstone, died from DLB.[45] Previously diagnosed with Parkinson's disease, American radio and television disc jockey and host Casey Kasem died in 2014 from DLB.[46] The Canadian singer Pierre Lalonde died from Parkinson's disease in 2016, but was found to have suffered from DLB.[47][48]
In the sports realm, Canadian ice hockey player Stan Mikita was diagnosed with DLB in January 2015,[49] and Jerry Sloan, American former professional basketball player and coach, announced in 2016 that he has Parkinson's disease and DLB.[50]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac McKeith IG, Boeve BF, Dickson DW, et al. (July 2017). "Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium". Neurology (Review). 89 (1): 88–100. doi:10.1212/WNL.0000000000004058. PMC 5496518. PMID 28592453.
- ^ a b c d e "What is Lewy Body Dementia?". NIA. 17 May 2017. Retrieved 22 March 2018.
{{cite web}}: Cite has empty unknown parameter:|1=(help) - ^ a b c d e f g h i j k l m n "NINDS Dementia With Lewy Bodies Information Page". NINDS. 2 November 2015. Archived from the original on 9 August 2016. Retrieved 3 October 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "Diagnosing Lewy Body Dementia". NIA. 29 Sep 2015. Archived from the original on 3 October 2016. Retrieved 3 October 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Hogan DB, Fiest KM, Roberts JI, Maxwell CJ, Dykeman J, Pringsheim T, Steeves T, Smith EE, Pearson D, Jetté N (April 2016). "The Prevalence and Incidence of Dementia with Lewy Bodies: a Systematic Review". Can J Neurol Sci. 43 Suppl 1: S83–95. doi:10.1017/cjn.2016.2. PMID 27307129.
- ^ a b c St Louis EK, Boeve BF (November 2017). "REM Sleep Behavior Disorder: Diagnosis, Clinical Implications, and Future Directions". Mayo Clin. Proc. (Review). 92 (11): 1723–1736. doi:10.1016/j.mayocp.2017.09.007. PMID 29101940.
- ^ "Common Symptoms". NIA. 29 July 2016. Archived from the original on 3 October 2016. Retrieved 3 October 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g h i j k l m n o Gomperts SN (April 2016). "Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia". Continuum (Minneap Minn) (Review). 22 (2 Dementia): 435–63. doi:10.1212/CON.0000000000000309. PMC 5390937. PMID 27042903.
- ^ Pezzoli S, Cagnin A, Bandmann O, Venneri A (July 2017). "Structural and Functional Neuroimaging of Visual Hallucinations in Lewy Body Disease: A Systematic Literature Review". Brain Sci (Review). 7 (7). doi:10.3390/brainsci7070084. PMC 5532597. PMID 28714891.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g Boot BP (2015). "Comprehensive treatment of dementia with Lewy bodies". Alzheimers Res Ther (Review). 7 (1): 45. doi:10.1186/s13195-015-0128-z. PMC 4448151. PMID 26029267.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h i j k l m n o Walker Z, Possin KL, Boeve BF, Aarsland D (October 2015). "Lewy body dementias". Lancet (Review). 386 (10004): 1683–97. doi:10.1016/S0140-6736(15)00462-6. PMC 5792067. PMID 26595642.
- ^ a b Kosaka K (2014). "Lewy body disease and dementia with Lewy bodies". Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. (Historical Review). 90 (8): 301–6. PMC 4275567. PMID 25311140.
- ^ a b c d e f g Weil RS, Lashley TL, Bras J, Schrag AE, Schott JM (2017). "Current concepts and controversies in the pathogenesis of Parkinson's disease dementia and Dementia with Lewy Bodies". F1000Res (Review). 6: 1604. doi:10.12688/f1000research.11725.1. PMC 5580419. PMID 28928962.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Goedert M, Jakes R, Spillantini MG (2017). "The Synucleinopathies: Twenty Years On". J Parkinsons Dis (Review). 7 (s1): S53–S71. doi:10.3233/JPD-179005. PMC 5345650. PMID 28282814.
- ^ a b c d e f g Karantzoulis S, Galvin JE (November 2011). "Distinguishing Alzheimer's disease from other major forms of dementia". Expert Rev Neurother (Review). 11 (11): 1579–91. doi:10.1586/ern.11.155. PMC 3225285. PMID 22014137.
- ^ St Louis EK, Boeve AR, Boeve BF (May 2017). "REM Sleep Behavior Disorder in Parkinson's Disease and Other Synucleinopathies". Mov. Disord. (Review). 32 (5): 645–658. doi:10.1002/mds.27018. PMID 28513079.
- ^ Boot BP (2015). "Comprehensive treatment of dementia with Lewy bodies". Alzheimers Res Ther (Review). 7 (1): 45. doi:10.1186/s13195-015-0128-z. PMC 4448151. PMID 26029267.
{{cite journal}}: Unknown parameter|lay-date=ignored (help); Unknown parameter|lay-source=ignored (help); Unknown parameter|lay-url=ignored (help)CS1 maint: unflagged free DOI (link) Original study here. - ^ a b Neef D, Walling AD (2006). "Dementia with Lewy Bodies: an Emerging Disease". American Family Physician (Review). 73 (7): 1223–29. PMID 16623209. Archived from the original on 2011-06-06.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: postscript (link) - ^ a b c d e Boot BP, McDade EM, McGinnis SM, Boeve BF (December 2013). "Treatment of dementia with lewy bodies". Curr Treat Options Neurol (Review). 15 (6): 738–64. doi:10.1007/s11940-013-0261-6. PMC 3913181. PMID 24222315.
- ^ Fisher A, Hanin I, Yoshinda M, ed. (1998). Progress in Alzheimer’s and Parkinson’s Diseases. New York: Springer. p. p. 53.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ [non-primary source needed] Presti, MF; Schmeichel, AM; Low, PA; Parisi, JE; Benarroch, EE (February 2014). "Degeneration of brainstem respiratory neurons in dementia with Lewy bodies". Sleep. 37 (2): 373–78. doi:10.5665/sleep.3418. PMC 3900631. PMID 24501436.
- ^ [non-primary source needed] Marla Gearing; Michael Lynn, MS; Suzanne S. Mirra, MD (February 1999), "Neurofibrillary Pathology in Alzheimer Disease With Lewy Bodies", Archives of Neurology, 56 (2): 203–8, doi:10.1001/archneur.56.2.203, PMID 10025425, archived from the original on 2010-03-28
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ [non-primary source needed] Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW (July 2008), "Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases.", J Neuropathol Exp Neurol., 67 (7): 649–56, doi:10.1097/NEN.0b013e31817d7a1d, PMC 2745052, PMID 18596548
- ^ Tousi B (October 2017). "Diagnosis and Management of Cognitive and Behavioral Changes in Dementia With Lewy Bodies". Curr Treat Options Neurol (Review). 19 (11): 42. doi:10.1007/s11940-017-0478-x. PMID 28990131.
- ^ "Diagnosing Dementia". NIA. 16 May 2017. Retrieved 22 March 2018.
{{cite web}}: Cite has empty unknown parameter:|1=(help) - ^ [non-primary source needed] Carey RJ, Pinheiro-Carrera M, Dai H, Tomaz C, Huston JP (2014-05-14). "L-DOPA and psychosis:". Biological Psychiatry. 38 (10). NIH.gov: 669–76. doi:10.1016/0006-3223(94)00378-5. PMID 8555378.
- ^ "Secondary parkinsonism". MedlinePlus Medical Encyclopedia. NIH.gov. Archived from the original on 2014-05-25. Retrieved 2014-06-10.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ [non-primary source needed] Ellul, J (2006). "The effects of commonly prescribed drugs in patients with Alzheimer's disease on the rate of deterioration". Journal of Neurology, Neurosurgery, and Psychiatry. 78 (3): 233–239. doi:10.1136/jnnp.2006.104034.
- ^ Velayudhan L, Ffytche D, Ballard C, Aarsland D (September 2017). "New Therapeutic Strategies for Lewy Body Dementias". Curr Neurol Neurosci Rep (Review). 17 (9): 68. doi:10.1007/s11910-017-0778-2. PMID 28741230.
- ^ [non-primary source needed] Aarsland, D; Ballard, C; Walker, Z; Bostrom, F; Alves, G; Kossakowski, K; Leroi, I; Pozo-Rodriguez, F; et al. (2009), "Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial", Lancet Neurology, 8 (7): 613–8, doi:10.1016/S1474-4422(09)70146-2, PMID 19520613
- ^ a b Mueller C, Ballard C, Corbett A, Aarsland D (May 2017). "The prognosis of dementia with Lewy bodies". Lancet Neurol (Review). 16 (5): 390–398. doi:10.1016/S1474-4422(17)30074-1. PMID 28342649.
- ^ [unreliable medical source?] Ferman, Tanis J.; Lewy Body Dementia Association (2007), Behavioral Challenges in Dementia with Lewy Bodies, from 'The Many Faces of Lewy Body Dementia' series at Coral Springs Medical Center, FL, archived from the original on 2017-02-13
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Cheng ST (August 2017). "Dementia Caregiver Burden: a Research Update and Critical Analysis". Curr Psychiatry Rep (Review). 19 (9): 64. doi:10.1007/s11920-017-0818-2. PMC 5550537. PMID 28795386.
- ^ Weintraub, Daniel; Hurtig, Howard I. (2007), "Presentation and Management of Psychosis in Parkinson's Disease and Dementia with Lewy Bodies", American Journal of Psychiatry, 164 (10): 1491–1498, doi:10.1176/appi.ajp.2007.07040715, PMC 2137166, PMID 17898337
- ^ Crystal, Howard A. (2008), "Dementia with Lewy Bodies", E-Medicine from WebMD, archived from the original on 2009-02-06
{{citation}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "First drug for Dementia with Lewy bodies approved". AsianScientist. 23 September 2014. Retrieved 22 March 2018.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Kosaka K, Oyanagi S, Matsushita M, Hori A (1976). "Presenile dementia with Alzheimer-, Pick- and Lewy-body changes". Acta Neuropathol. 36 (3): 221–233. doi:10.1007/bf00685366. PMID 188300.
- ^ "Robin Williams' widow blames Lewy body dementia". CNN. Archived from the original on 2015-11-04. Retrieved 2015-11-04.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Williams SS (September 2016). "The terrorist inside my husband's brain". Neurology. 87 (13): 1308–11. doi:10.1212/WNL.0000000000003162. PMID 27672165.
- ^ McKeith, Ian. "Robin Williams had dementia with Lewy Bodies -- so, what is it and why has it been eclipsed by Alzheimer's?". The Conversation. Archived from the original on 2016-11-04. Retrieved 2016-11-01.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b {Sahlas DJ (June 2003). "Dementia with Lewy bodies and the neurobehavioral decline of Mervyn Peake". Arch. Neurol. 60 (6): 889–92. doi:10.1001/archneur.60.6.889. PMID 12810496.
- ^ Shaw, David and Mitchell Landsberg (February 27, 2006). "L.A. Icon Otis Chandler Dies at 78". The Los Angeles Times. Archived from the original on April 26, 2007. Retrieved July 23, 2008.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Carlson, Michael (July 24, 2008). "Obituary: Estelle Getty". theguardian.com. Archived from the original on September 2, 2013. Retrieved October 13, 2013.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Dangremond, Sam (May 23, 2017). "Actress and Philanthropist Dina Merrill Dies at 93". Town and Country Magazine. Retrieved March 22, 2018.
- ^ Woo, Elaine (June 24, 2015). "Don Featherstone dies at 79; creator of the plastic pink flamingo". Los Angeles Times. Retrieved March 22, 2018.
- ^ Caffrey, Jane (April 18, 2016). "Casey Kasem and a lesson about end-of-life care". CNN. Retrieved March 22, 2018.
- ^ "Pierre Lalonde est décédé à l'âge de 75 ans". Le Journal de Montréal. Retrieved 2016-06-22.
- ^ Belanger, Cedric (June 22, 2016). "Pierre Lalonde souffrait aussi de la démence à corps de Lewy". Le Journal de Montréal. Retrieved March 22, 2018.
- ^ Kuc, Chris (June 15, 2015). "For Stan Mikita, all the Blackhawks memories are gone". Chicago Tribune. Archived from the original on 16 June 2015. Retrieved March 22, 2018.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Hall of Fame coach Jerry Sloan battling Parkinson's disease, Lewy body dementia". espn.go.com. April 6, 2016. Archived from the original on April 8, 2016. Retrieved April 7, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
External links
- Video of updated diagnostic criteria from the Lewy Body Dementia Association, with Ian McKeith.
