Stem cell: Difference between revisions
m sp (2): a embryonic→an embryonic, in vitro→''in vitro'' |
→Treatments: Sounds like propaganda against adult stem cell research. Who removed the information about Adult Stem Cell therapy for leukemia? |
||
| Line 55: | Line 55: | ||
==Treatments== |
==Treatments== |
||
{{main|Stem cell treatments}} |
{{main|Stem cell treatments}} |
||
Medical researchers believe that stem cell research has the potential to change the face of human disease. A number of current treatments already exist, although the majority of them are not commonly used because they tend to be experimental and not very cost-effective. Medical researchers anticipate being able to use technologies derived from stem cell research to treat [[cancer]], [[parkinson's disease]], [[spinal cord injuries]], and [[muscle]] damage, amongst a number of other diseases, impairments and conditions. |
Medical researchers believe that stem cell research has the potential to change the face of human disease. A number of current treatments already exist, although the majority of them are not commonly used because they tend to be experimental and not very cost-effective.{{fact}} Medical researchers anticipate being able to use technologies derived from stem cell research to treat [[cancer]], [[parkinson's disease]], [[spinal cord injuries]], and [[muscle]] damage, amongst a number of other diseases, impairments and conditions. |
||
However, there still exists a great deal of social and scientific uncertainty surrounding stem cell research, which could possibly be overcome through public debate and future research. |
However, there still exists a great deal of social and scientific uncertainty surrounding stem cell research, which could possibly be overcome through public debate and future research. |
||
Revision as of 01:43, 18 January 2007
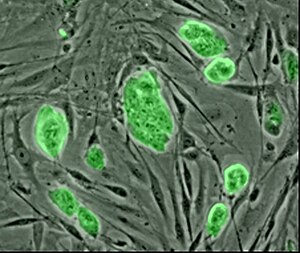
Stem cells are primal cells common to all multi-cellular organisms that retain the ability to renew themselves through cell division and can differentiate into a wide range of specialized cell types. Research in the human stem cell field grew out of findings by Canadian scientists Ernest A. McCulloch and James E. Till in the 1960s.[1][2]
The three broad categories of mammalian stem cells exist: embryonic stem cells, derived from blastocysts, adult stem cells, which are found in adult tissues, and cord blood stem cells, which are found in the umbilical cord. In a developing embryo, stem cells are able to differentiate into all of the specialized embryonic tissues. In adult organisms, stem cells and progenitor cells act as a repair system for the body, replenishing specialized cells.
As stem cells can be readily grown and transformed into specialised tissues such as muscles or nerves through cell culture, their use in medical therapies has been proposed. In particular, embryonic cell lines, autologous embryonic stem cells generated through therapeutic cloning, and highly plastic adult stem cells from the umbilical cord blood or bone marrow are touted as promising candidates.[3]
Stem cell properties
Defining properties
The rigorous definition of a stem cell requires that it possesses two properties:
- Self-renewal - the ability to go through numerous cycles of cell division while maintaining the undifferentiated state.
- Unlimited potency - the capacity to differentiate into any mature cell type. In a strict sense, this makes stem cells either totipotent or pluripotent, although some multipotent and/or unipotent progenitor cells are sometimes referred to as stem cells.
These properties can be illustrated in vitro, using methods such as clonogenic assays, where the progeny of single cell is characterized.[4][5] However, in vitro culture conditions can alter the behavior of cells, making it unclear whether the cells will behave in a similar manner in vivo. Considerable debate exists whether some proposed adult cell populations are truly stem cells.
Potency definitions

Potency specifies the differentiation potential (the potential to differentiate into different cell types) of the stem cell.
- Totipotent stem cells are produced from the fusion of an egg and sperm cell. Cells produced by the first few divisions of the fertilized egg are also totipotent. These cells can differentiate into embryonic and extraembryonic cell types.
- Pluripotent stem cells are the descendants of totipotent cells and can differentiate into cells derived from the three germ layers.
- Multipotent stem cells can produce only cells of a closely related family of cells (e.g. hematopoietic stem cells differentiate into red blood cells, white blood cells, platelets, etc.).
- Unipotent cells can produce only one cell type, but have the property of self-renewal which distinguishes them from non-stem cells.
Embryonic stem cells
Embryonic stem cell lines (ES cell lines) are cultures of cells derived from the epiblast tissue of the inner cell mass (ICM) of a blastocyst. A blastocyst is an early stage embryo - approximately 4 to 5 days old in humans and consisting of 50-150 cells. ES cells are pluripotent, and give rise during development to all derivatives of the three primary germ layers: ectoderm, endoderm and mesoderm. In other words, they can develop into each of the more than 200 cell types of the adult body when given sufficient and necessary stimulation for a specific cell type. They do not contribute to the extra-embryonic membranes or the placenta.
When given no stimuli for differentiation, ES cells will continue to divide in vitro and each daughter cell will remain pluripotent. The pluripotency of ES cells has been rigorously demonstrated in vitro and in vivo, thus they can be indeed classified as stem cells.
Because of their unique combined abilities of unlimited expansion and pluripotency, embryonic stem cells are a potential source for regenerative medicine and tissue replacement after injury or disease. To date, no approved medical treatments have been derived from embryonic stem cell research. This is not surprising considering that many nations currently have moratoria on either ES cell research or the production of new ES cell lines.
Adult stem cells

Adult stem cells are undifferentiated cells found throughout the body that divide to replenish dying cells and regenerate damaged tissues. Also known as somatic (from Greek Σωματικóς, of the body) stem cells, they can be found in children, as well as adults.
A great deal of adult stem cell research has focused on clarifying their capacity to divide or self-renew indefinitely and their differentiation potential.[6] Many adult stem cells may be better classified as progenitor cells, due to their limited capacity for cellular differentiation.
Nevertheless, specific multipotent or even unipotent adult progenitors may have potential utility in regenerative medicine. The use of adult stem cells in research and therapy is not as controversial as embryonic stem cells, because the production of adult stem cells does not require the destruction of an embryo. In contrast with the embryonic stem cell research, more US government funding has been provided for adult stem cell research. Adult stem cells can be isolated from a tissue sample obtained from an adult. They have mainly been studied in humans and model organisms such as mice and rats.
Lineage
To ensure self-renewal, stem cells undergo two types of cell division (see Stem cell division and differentiation diagram). Symmetric division gives rise to two identical daughter cells both endowed with stem cell properties. Asymmetric division, on the other hand, produces only one stem cell and a progenitor cell with limited self-renewal potential. Progenitors can go through several rounds of cell division before terminally differentiating into a mature cell. It is possible that the molecular distinction between symmetric and asymmetric divisions lies in differential segregation of cell membrane proteins (such as receptors) between the daughter cells, however, there is no evidence for this mechanism.
An alternative theory is that stem cells remain undifferentiated from environmental cues in their particular niche. Stem cells differentiate when they leave that niche or no longer receive those signals. Studies in Drosophila germarium have identified the signals dpp and adherins junctions that prevent germarium stem cells from differentiating[7][8].
The signals that lead to reprogramming of cells to an embryonic-like state are also being investigated. These signal pathways include several transcription factors including the oncogene c-Myc. Initial studies indicate that transformation of mice cells with a combination of these anti-differentiation signals can reverse differentiation and may allow adult cells to become pluripotent.[9] However, need to transform these cells with an oncogene may prevent the use of this approach in therapy.
Treatments
Medical researchers believe that stem cell research has the potential to change the face of human disease. A number of current treatments already exist, although the majority of them are not commonly used because they tend to be experimental and not very cost-effective.[citation needed] Medical researchers anticipate being able to use technologies derived from stem cell research to treat cancer, parkinson's disease, spinal cord injuries, and muscle damage, amongst a number of other diseases, impairments and conditions. However, there still exists a great deal of social and scientific uncertainty surrounding stem cell research, which could possibly be overcome through public debate and future research.
Stem cells, however, are already used extensively in research, and some scientists do not see cell therapy as the first goal of the research, but see the investigation of stem cells as a goal worthy in itself. [10].
Controversy surrounding stem cell research
There exists a widespread controversy over stem cell research that emanates from the techniques used in the creation and usage of stem cells. Embryonic stem cell research is particularly controversial because, with the present state of technology, starting a stem cell line requires the destruction of a human embryo and/or therapeutic cloning. Opponents of the research argue that this practice is a slippery slope to reproductive cloning and tantamount to the instrumentalization of a human being. Contrarily, medical researchers in the field argue that it is necessary to pursue embryonic stem cell research because the resultant technologies are expected to have significant medical potential, and that the embryos used for research are only those meant for destruction anyway (as a product of invitro fertilisation). This in turn, conflicts with opponents holding an anti-abortion stance, who argue that an embryo is a human being and therefore entitled to dignity even if legally slated for destruction. The ensuing debate has prompted authorities around the world to seek regulatory frameworks and highlighted the fact that stem cell research represents a social and ethical challenge.
Key events in stem cell research
- 1960s - Joseph Altman and Gopal Das present evidence of adult neurogenesis, ongoing stem cell activity in the brain; their reports contradict Cajal's "no new neurons" dogma and are largely ignored
- 1963 - McCulloch and Till illustrate the presence of self-renewing cells in mouse bone marrow
- 1968 - Bone marrow transplant between two siblings successfully treats SCID
- 1978 - Haematopoietic stem cells are discovered in human cord blood
- 1981 - Mouse embryonic stem cells are derived from the inner cell mass
- 1992 - Neural stem cells are cultured in vitro as neurospheres
- 1995 - President Bill Clinton signs into law the Dickey Amendment which prohibited Federally appropriated funds to be used for research where human embryos would be either created or destroyed.
- 1997 - Leukemia is shown to originate from a haematopoietic stem cell, the first direct evidence for cancer stem cells
- 1998 - James Thomson and coworkers derive the first human embryonic stem cell line at the University of Wisconsin-Madison.
- 2000s - Several reports of adult stem cell plasticity are published
- 2003 - Dr. Songtao Shi of NIH discovers new source of adult stem cells in children's primary teeth[11]
- 02 November, 2004 - California voters approve Proposition 71, which provides $3 billion in state funds over ten years to human embryonic stem cell research.
- 2004-2005 - Korean researcher Hwang Woo-Suk claims to have created several human embryonic stem cell lines from unfertilised human oocytes. The lines are later shown to be fabricated.
- 2005 - Researchers at Kingston University in England claim to have discovered a third category of stem cell, dubbed cord-blood-derived embryoniclike stem cells (CBEs), derived from umbilical cord blood. The group claims these cells are able to differentiate into more types of tissue than adult stem cells.
- 2001-2006 - President George W. Bush endorses the Congress in providing federal funding for embryonic stem cell research of approximately $100 million as well as $250 million dollars for research on adult and animal stem cells. He also enacts laws that restrict federally-funded stem cell research on embryonic stem cells to the already derived cell lines.
- 5 May, 2006 - Rick Santorum introduces bill number S. 2754, or the Alternative Pluripotent Stem Cell Therapies Enhancement Act. into the Senate
- 18 July, 2006 - The U.S. Senate passes the Stem Cell Research Enhancement Act H.R. 810, and votes down Senator Santorum's S.2754.
- 19 July, 2006 - President George W. Bush vetoes H.R. 810 (Stem Cell Research Enhancement Act), a bill that would have reversed the Clinton-era law which made it illegal for Federal money to be used for research where stem cells are derived from the destruction of an embryo.
- August 2006 - Cell Journal publishes Kazutoshi Takahashi and Shinya Yamanaka, Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors
- 07 November, 2006 - The people of the state of Missouri vote to protect the possibility of Stem Cell research in their state.
- 07 January, 2007 - Scientists at Wake Forest University led by Dr. Anthony Atala and Harvard University report discovery of a new type of stem cell in amniotic fluid.[1] This may potentially provide an alternative to embryonic stem cells for use in research and therapy. [2]
See also
References
- ^ Becker AJ, McCulloch EA, Till JE (1963). "Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells". Nature. 197: 452–4. PMID 13970094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Siminovitch L, McCulloch EA, Till JE (1963). "The distribution of colony-forming cells among spleen colonies". Journal of Cellular and Comparative Physiology. 62: 327–36. PMID 14086156.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tuch B (2006). "Stem cells--a clinical update". Aust Fam Physician. 35 (9): 719–21. PMID 16969445.
- ^ Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA (1974). "Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method". Exp Hematol. 2 (2): 83–92. PMID 4455512.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Friedenstein AJ, Gorskaja JF, Kulagina NN (1976). "Fibroblast precursors in normal and irradiated mouse hematopoietic organs". Exp Hematol. 4 (5): 267–74. PMID 976387.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gardner RL (2002). "Stem cells: potency, plasticity and public perception". Journal of Anatomy. 200 (3): 277–82. PMID 12033732.
- ^ Xie T, Spradling A (1998). "decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary". Cell. 94 (2): 251–60. PMID 9695953.
- ^ Song X, Zhu C, Doan C, Xie T (2002). "Germline stem cells anchored by adherens junctions in the Drosophila ovary niches". Science. 296 (5574): 1855–7. PMID 12052957.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Takahashi K, Yamanaka S (2006). "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell. 126 (4): 663–76. PMID 16904174.
- ^ Wade N (2006-08-14). "Some Scientists See Shift in Stem Cell Hopes". New York Times. Retrieved 2006-12-28.
- ^ Shostak S (2006). "(Re)defining stem cells". Bioessays. 28 (3): 301–8. PMID 16479584.
External links
- General
- Peer-reviewed journals
