Regulation of gene expression: Difference between revisions
Copy Editing |
|||
| Line 4: | Line 4: | ||
[[File:Gene expression control.png|thumb|Diagram showing at which stages in the DNA-mRNA-protein pathway expression can be controlled]] |
[[File:Gene expression control.png|thumb|Diagram showing at which stages in the DNA-mRNA-protein pathway expression can be controlled]] |
||
'''Regulation of gene expression''' includes a wide range of mechanisms |
'''Regulation of gene expression''' includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific [[gene product]]s ([[protein]] or [[RNA]]), and is informally termed ''gene regulation''. Sophisticated programs of [[gene expression]] are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from [[Transcriptional regulation|transcriptional initiation]], to [[RNA processing]], and to the [[post-translational modification]] of a protein. |
||
Gene regulation is essential for [[viruses]], [[prokaryote]]s and [[eukaryote]]s |
Gene regulation is essential for [[viruses]], [[prokaryote]]s and [[eukaryote]]s as it increases the versatility and adaptability of an [[organism]] by allowing the cell to express protein when needed. Although as early as 1951 [[Barbara McClintock]] showed interaction between two genetic loci, Activator (''Ac'') and Dissociator (''Ds''), in the color formation of maize seeds, the first discovery of a gene regulation system is widely considered to be the identification in 1961 of the [[lac operon|''lac'' operon]], discovered by [[Jacques Monod]], in which some enzymes involved in [[lactose]] metabolism are expressed by the genome of ''[[Escherichia coli|E. coli]]'' only in the presence of lactose and absence of glucose. |
||
Furthermore, in multicellular organisms, gene regulation drives the processes of [[cellular differentiation]] and [[morphogenesis]], leading to the creation of different cell types that possess different gene expression profiles, and hence produce different proteins/have different ultrastructures that suit them to their functions (though they all possess the genotype, which follows the same [[genome]] sequence). |
Furthermore, in multicellular organisms, gene regulation drives the processes of [[cellular differentiation]] and [[morphogenesis]], leading to the creation of different cell types that possess different gene expression profiles, and hence produce different proteins/have different ultrastructures that suit them to their functions (though they all possess the genotype, which follows the same [[genome]] sequence). |
||
Revision as of 14:21, 14 February 2014
- Gene modulation redirects here. For information on therapeutic regulation of gene expression, see therapeutic gene modulation.
- For vocabulary, see Glossary of gene expression terms
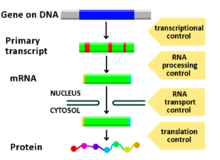
Regulation of gene expression includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products (protein or RNA), and is informally termed gene regulation. Sophisticated programs of gene expression are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from transcriptional initiation, to RNA processing, and to the post-translational modification of a protein.
Gene regulation is essential for viruses, prokaryotes and eukaryotes as it increases the versatility and adaptability of an organism by allowing the cell to express protein when needed. Although as early as 1951 Barbara McClintock showed interaction between two genetic loci, Activator (Ac) and Dissociator (Ds), in the color formation of maize seeds, the first discovery of a gene regulation system is widely considered to be the identification in 1961 of the lac operon, discovered by Jacques Monod, in which some enzymes involved in lactose metabolism are expressed by the genome of E. coli only in the presence of lactose and absence of glucose.
Furthermore, in multicellular organisms, gene regulation drives the processes of cellular differentiation and morphogenesis, leading to the creation of different cell types that possess different gene expression profiles, and hence produce different proteins/have different ultrastructures that suit them to their functions (though they all possess the genotype, which follows the same genome sequence).
Regulated stages of gene expression
Any step of gene expression may be modulated, from the DNA-RNA transcription step to post-translational modification of a protein. The following is a list of stages where gene expression is regulated, the most extensively utilised point is Transcription Initiation:
- Chromatin domains
- Transcription
- Post-transcriptional modification
- RNA transport
- Translation
- mRNA degradation
Modification of DNA
In eukaryotes, the accessibility of large regions of DNA can depend on its chromatin structure, which can be altered as a result of histone modifications directed by DNA methylation, ncRNA, or DNA-binding protein. Hence these modifications may up or down regulate the expression of a gene. Certain of these modifications that regulate gene expression are inheritable and are referred to as epigenetic regulation.
Structural
Transcription of DNA is dictated by its structure. In general, the density of its packing is indicative of the frequency of transcription. Octameric protein complexes called nucleosomes are responsible for the amount of supercoiling of DNA, and these complexes can be temporarily modified by processes such as phosphorylation or more permanently modified by processes such as methylation. Such modifications are considered to be responsible for more or less permanent changes in gene expression levels.[1]
Chemical
Methylation of DNA is a common method of gene silencing. DNA is typically methylated by methyltransferase enzymes on cytosine nucleotides in a CpG dinucleotide sequence (also called "CpG islands" when densely clustered). Analysis of the pattern of methylation in a given region of DNA (which can be a promoter) can be achieved through a method called bisulfite mapping. Methylated cytosine residues are unchanged by the treatment, whereas unmethylated ones are changed to uracil. The differences are analyzed by DNA sequencing or by methods developed to quantify SNPs, such as Pyrosequencing (Biotage) or MassArray (Sequenom), measuring the relative amounts of C/T at the CG dinucleotide. Abnormal methylation patterns are thought to be involved in oncogenesis.[2]
Histone acetylation is also an important process in transcription. Histone acetyltransferase enzymes (HATs) such as CREB-binding protein also dissociate the DNA from the histone complex, allowing transcription to proceed. Often, DNA methylation and histone deacetylation work together in gene silencing. The combination of the two seems to be a signal for DNA to be packed more densely, lowering gene expression.[citation needed]
Regulation of transcription
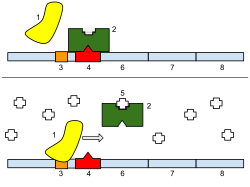
Regulation of transcription thus controls when transcription occurs and how much RNA is created. Transcription of a gene by RNA polymerase can be regulated by at least five mechanisms:
- Specificity factors alter the specificity of RNA polymerase for a given promoter or set of promoters, making it more or less likely to bind to them (i.e., sigma factors used in prokaryotic transcription).
- Repressors bind to the Operator, coding sequences on the DNA strand that are close to or overlapping the promoter region, impeding RNA polymerase's progress along the strand, thus impeding the expression of the gene.The image to the right demonstrates regulation by a repressor in the lac operon.
- General transcription factors position RNA polymerase at the start of a protein-coding sequence and then release the polymerase to transcribe the mRNA.
- Activators enhance the interaction between RNA polymerase and a particular promoter, encouraging the expression of the gene. Activators do this by increasing the attraction of RNA polymerase for the promoter, through interactions with subunits of the RNA polymerase or indirectly by changing the structure of the DNA.
- Enhancers are sites on the DNA helix that are bound to by activators in order to loop the DNA bringing a specific promoter to the initiation complex. Enhancers are much more common in eukaryote than prokaryotes, where only a few examples exist (to date).[3]
- Silencers are regions of DNA sequences that, when bound by particular transcription factors, can silence expression of the gene.
Post-transcriptional regulation
After the DNA is transcribed and mRNA is formed, there must be some sort of regulation on how much the mRNA is translated into proteins. Cells do this by modulating the capping, splicing, addition of a Poly(A) Tail, the sequence-specific nuclear export rates, and, in several contexts, sequestration of the RNA transcript. These processes occur in eukaryotes but not in prokaryotes. This modulation is a result of a protein or transcript that, in turn, is regulated and may have an affinity for certain sequences.
Regulation of translation
The translation of mRNA can also be controlled by a number of mechanisms, mostly at the level of initiation. Recruitment of the small ribosomal subunit can indeed be modulated by mRNA secondary structure, antisense RNA binding, or protein binding. In both prokaryotes and eukaryotes, a large number of RNA binding proteins exist, which often are directed to their target sequence by the secondary structure of the transcript, which may change depending on certain conditions, such as temperature or presence of a ligand (aptamer). Some transcripts act as ribozymes and self-regulate their expression.
Examples of gene regulation
- Enzyme induction is a process in which a molecule (e.g., a drug) induces (i.e., initiates or enhances) the expression of an enzyme.
- The induction of heat shock proteins in the fruit fly Drosophila melanogaster.
- The Lac operon is an interesting example of how gene expression can be regulated.
- Viruses, despite having only a few genes, possess mechanisms to regulate their gene expression, typically into an early and late phase, using collinear systems regulated by anti-terminators (lambda phage) or splicing modulators (HIV).
Developmental biology
A large number of studied regulatory systems come from developmental biology. Examples include:
- The colinearity of the Hox gene cluster with their nested antero-posterior patterning
- It has been speculated that pattern generation of the hand (digits - interdigits) The gradient of Sonic hedgehog (secreted inducing factor) from the zone of polarizing activity in the limb, which creates a gradient of active Gli3, which activates Gremlin, which inhibits BMPs also secreted in the limb, resulting in the formation of an alternating pattern of activity as a result of this reaction-diffusion system.
- Somitogenesis is the creation of segments (somites) from a uniform tissue (Pre-somitic Mesoderm, PSM). They are formed sequentially from anterior to posterior. This is achieved in amniotes possibly by means of two opposing gradients, Retinoic acid in the anterior (wavefront) and Wnt and Fgf in the posterior, coupled to an oscillating pattern (segmentation clock) composed of FGF + Notch and Wnt in antiphase.[4]
- Sex determination in the soma of a Drosophila requires the sensing of the ratio of autosomal genes to sex chromosome-encoded genes, which results in the production of sexless splicing factor in females, resulting in the female isoform of doublesex.[5]
Circuitry
Up-regulation and down-regulation
Up-regulation is a process that occurs within a cell triggered by a signal (originating internal or external to the cell), which results in increased expression of one or more genes and as a result the protein(s) encoded by those genes. On the converse, down-regulation is a process resulting in decreased gene and corresponding protein expression.
- Up-regulation occurs, for example, when a cell is deficient in some kind of receptor. In this case, more receptor protein is synthesized and transported to the membrane of the cell and, thus, the sensitivity of the cell is brought back to normal, reestablishing homeostasis.
- Down-regulation occurs, for example, when a cell is overstimulated by a neurotransmitter, hormone, or drug for a prolonged period of time, and the expression of the receptor protein is decreased in order to protect the cell (see also tachyphylaxis).
Inducible vs. repressible systems
Gene Regulation can be summarized by the response of the respective system:
- Inducible systems - An inducible system is off unless there is the presence of some molecule (called an inducer) that allows for gene expression. The molecule is said to "induce expression". The manner by which this happens is dependent on the control mechanisms as well as differences between prokaryotic and eukaryotic cells.
- Repressible systems - A repressible system is on except in the presence of some molecule (called a corepressor) that suppresses gene expression. The molecule is said to "repress expression". The manner by which this happens is dependent on the control mechanisms as well as differences between prokaryotic and eukaryotic cells.
Theoretical circuits
- Repressor/Inducer: an activation of a sensor results in the change of expression of a gene
- negative feedback: the gene product downregulates its own production directly or indirectly, which can result in
- keeping transcript levels constant/proportional to a factor
- inhibition of run-away reactions when coupled with a positive feedback loop
- creating an oscillator by taking advantage in the time delay of transcription and translation, given that the mRNA and protein half-life is shorter
- positive feedback: the gene product upregulates its own production directly or indirectly, which can result in
- signal amplification
- bistable switches when two genes inhibit each other and both have positive feedback
- pattern generation
Methods
In general, most experiments investigating differential expression used whole cell extracts of RNA, called steady-state levels, to determine which genes changed and by how much they did. These are, however, not informative of where the regulation has occurred and may actually mask conflicting regulatory processess (see post-transcriptional regulation), but it is still the most commonly analysed (quantitative PCR and DNA microarray).
When studying gene expression, there are several methods to look at the various stages. In eukaryotes these include:
- The local chromatin environment of the region can be determined by ChIP-chip analysis by pulling down RNA Polymerase II, Histone 3 modifications, Trithorax-group protein, Polycomb-group protein, or any other DNA-binding element to which a good antibody is available.
- Epistatic interactions can be investigated by synthetic genetic array analysis
- Due to post-transcriptional regulation, transcription rates and total RNA levels differ significantly. To measure the transcription rates nuclear run-on assays can be done and newer high-throughput methods are being developed, using thiol labelling instead of radioactivity.[6]
- Only 5% of the RNA polymerised in the nucleus actually exits,[7] and not only introns, abortive products, and non-sense transcripts are degradated. Therefore, the differences in nuclear and cytoplasmic levels can be see by separating the two fractions by gentle lysis.[8]
- Alternative splicing can be analysed with a splicing array or with a tiling array (see DNA microarray).
- All in vivo RNA is complexed as RNPs. The quantity of transcripts bound to specific protein can be also analysed by RIP-Chip. For example, DCP2 will give an indication of sequestered protein; ribosome-bound gives and indication of transcripts active in transcription (although it should be noted that a more dated method, called polysome fractionation, is still popular in some labs)
- Protein levels can be analysed by Mass spectrometry, which can be compared only to quantitative PCR data, as microarray data is relative and not absolute.
- RNA and protein degradation rates are measured by means of transcription inhibitors (actinomycin D or α-amanitin) or translation inhibitors (Cycloheximide), respectively.
See also
- Enhancer (genetics)
- Artificial transcription factors (small molecules that mimic transcription factor protein)
- Cellular model
- Conserved non-coding DNA sequence
- Spatiotemporal gene expression
Notes and references
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1186/gb-2011-12-1-r10, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1186/gb-2011-12-1-r10instead. - ^ Vertino PM, Spillare EA, Harris CC, Baylin SB (April 1993). "Altered chromosomal methylation patterns accompany oncogene-induced transformation of human bronchial epithelial cells" (PDF). Cancer Res. 53 (7): 1684–9. PMID 8453642.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Austin S, Dixon R (June 1992). "The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent". EMBO J. 11 (6): 2219–28. PMC 556689. PMID 1534752.
- ^ Dequéant ML, Pourquié O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008 May;9(5):370-82. PMID 18414404
- ^ Gilbert SF (2003). Developmental biology, 7th ed., Sunderland, Mass: Sinauer Associates, 65–6. ISBN 0-87893-258-5.
- ^ Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG (2005). "Control of gene expression during T cell activation: alternate regulation of mRNA transcription and mRNA stability". BMC Genomics. 6: 75. doi:10.1186/1471-2164-6-75. PMC 1156890. PMID 15907206.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Jackson DA, Pombo A, Iborra F (2000). "The balance sheet for transcription: an analysis of nuclear RNA metabolism in mammalian cells". FASEB J. 14 (2): 242–54. PMID 10657981.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schwanekamp JA, Sartor MA, Karyala S, Halbleib D, Medvedovic M, Tomlinson CR (2006). "Genome-wide analyses show that nuclear and cytoplasmic RNA levels are differentially affected by dioxin". Biochim. Biophys. Acta. 1759 (8–9): 388–402. doi:10.1016/j.bbaexp.2006.07.005. PMID 16962184.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Latchman, David S. (2005). Gene regulation: a eukaryotic perspective. Psychology Press. ISBN 978-0-415-36510-9.
External links
- Gene Regulation Info -- manually curated lists of resources, reviews, community discussions
- Cellular Darwinism
- Regulation of Gene Expression at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
