High-altitude pulmonary edema: Difference between revisions
Ozzie10aaaa (talk | contribs) |
Ozzie10aaaa (talk | contribs) →Signs and symptoms: MEDMOS Tag: use of predatory open access journal |
||
| Line 49: | Line 49: | ||
* [[Tachypnea]] (rapid breathing) |
* [[Tachypnea]] (rapid breathing) |
||
* [[Tachycardia]] (rapid heart rate) |
* [[Tachycardia]] (rapid heart rate) |
||
==Risk factors== |
|||
There are multiple factors that can contribute to the development of HAPE, including gender (male), genetic factors, prior development of HAPE, ascent rate, cold exposure, peak altitude, intensity of physical exertion, and certain underlying medical conditions (eg, pulmonary hypertension).<ref name=":1" /><ref name=":0" /> Anatomic abnormalities that are predisposing include [[Pulmonary atresia|congenital absence of pulmonary artery]], and left-to-right [[Acyanotic heart defect|intracardiac shunts]] (eg, atrial and ventricular septal defects), both of which increase pulmonary blood flow.<ref name=":1" /><ref name=":0" /> HAPE-susceptible (HAPE-s) individuals were also found to be four times more likely to have a [[Foramen ovale (heart)|patent foramen ovale]] (PFO) than those who were HAPE-resistant.<ref name=":1" /> There is currently no indication or recommendation for people with PFO to pursue closure prior to extreme altitude exposure.<ref name=":1" /> |
|||
In studies performed at sea level, HAPE-s people were found to have exaggerated circulatory response to both hypoxia at rest and during exercise.<ref name=":1" /> In these individuals, the [[pulmonary artery pressure]] (PAP) and [[pulmonary vascular resistance]] (PVR) were shown to be abnormally high.<ref name=":1" /> [[Microneurography|Microneurographic]] recordings in these individuals developed a direct link between PAP rise and [[sympathetic nervous system]] over-activation, which could explain the exaggerated response to hypoxia in these persons.<ref name=":1" /> |
|||
[[Endothelium|Endothelial tissue]] dysfunction has also been linked to development of HAPE, including reduced synthesis of [[Nitric oxide synthase|NO]] (a potent [[Vasodilation|vasodilator]]), increased levels of [[endothelin]] (a potent [[Vasoconstriction|vasconstrictor]]), and an impaired ability to transport sodium and water across the [[epithelium]] and out of the [[alveoli]].<ref name=":1" /> |
|||
Current data on the genetic basis for HAPE susceptibility is conflicting and interpretation is difficult. Genes implicated in the development of HAPE include those in the [[Renin–angiotensin system|renin-angiotensin system]] (RAS), [[Nitric oxide synthase|NO pathway]], and [[Hypoxia-inducible factors|hypoxia-inducible factor pathway]] (HIF), among others.<ref name=":1" /><ref name=":2">{{Cite journal|last=Anamika Gangwar|first=Subhojit Paul|date=2015|title=High Altitude Pulmonary Edema: An Update on Omics Data and Redefining Susceptibility|url=|journal=Journal of Proteomics & Bioinformatics|volume=08|issue=06|pages=|doi=10.4172/jpb.1000359|via=}}</ref> Future [[Genetic testing|genomic testing]] could provide a much clearer picture of the genetic factors that contribute to HAPE.<ref name=":1" /><ref name=":2" /> |
|||
==Pathophysiology== |
==Pathophysiology== |
||
Revision as of 19:39, 26 May 2019
| High-Altitude Pulmonary Edema (HAPE) | |
|---|---|
| Other names | High-altitude pulmonary oedema (HAPO) |
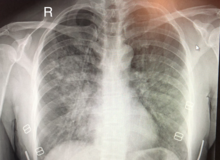 | |
| Plain chest radiograph (x-ray) of a female patient with HAPE showing characteristic patchy alveolar infiltrates with right middle lobe predominance. | |
| Specialty | Emergency medicine |
High-altitude pulmonary edema (HAPE) is a life-threatening form of non-cardiogenic pulmonary edema (fluid accumulation in the lungs) that occurs in otherwise healthy mountaineers at altitudes typically above 2,500 meters (8,200 ft).[1] However, cases have also been reported at lower altitudes (between 1,500–2,500 metres or 4,900–8,200 feet) in highly vulnerable subjects.
Classically, HAPE occurs in persons normally living at low altitude who travel or ascend to an altitude above 2,500 meters (8,200 feet).[2] Re-entry HAPE is also an entity that has been described in persons who normally live at high altitude but who develop pulmonary edema after returning from a stay at low altitude.[2]
There are many factors that can make a person more susceptible to developing HAPE, including genetic factors, but detailed understanding is lacking and currently under investigation. HAPE remains the major cause of death related to high-altitude exposure, with a high mortality rate in the absence of adequate emergency treatment.[2]
Signs and symptoms
Physiological and symptomatic changes often vary according to the altitude involved.[3]
The Lake Louise Consensus Definition for high-altitude pulmonary edema has set widely used criteria for defining HAPE symptoms.[4]
In the presence of a recent gain in altitude, the presence of the following:
Symptoms: at least two of:
- Shortness of breath at rest
- Cough
- Weakness or decreased exercise performance
- Chest tightness or congestion
Signs: at least two of:
- Crackles or wheezing (while breathing) in at least one lung field
- Central blue skin color
- Tachypnea (rapid breathing)
- Tachycardia (rapid heart rate)
Risk factors
There are multiple factors that can contribute to the development of HAPE, including gender (male), genetic factors, prior development of HAPE, ascent rate, cold exposure, peak altitude, intensity of physical exertion, and certain underlying medical conditions (eg, pulmonary hypertension).[5][2] Anatomic abnormalities that are predisposing include congenital absence of pulmonary artery, and left-to-right intracardiac shunts (eg, atrial and ventricular septal defects), both of which increase pulmonary blood flow.[5][2] HAPE-susceptible (HAPE-s) individuals were also found to be four times more likely to have a patent foramen ovale (PFO) than those who were HAPE-resistant.[5] There is currently no indication or recommendation for people with PFO to pursue closure prior to extreme altitude exposure.[5]
In studies performed at sea level, HAPE-s people were found to have exaggerated circulatory response to both hypoxia at rest and during exercise.[5] In these individuals, the pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) were shown to be abnormally high.[5] Microneurographic recordings in these individuals developed a direct link between PAP rise and sympathetic nervous system over-activation, which could explain the exaggerated response to hypoxia in these persons.[5]
Endothelial tissue dysfunction has also been linked to development of HAPE, including reduced synthesis of NO (a potent vasodilator), increased levels of endothelin (a potent vasconstrictor), and an impaired ability to transport sodium and water across the epithelium and out of the alveoli.[5]
Current data on the genetic basis for HAPE susceptibility is conflicting and interpretation is difficult. Genes implicated in the development of HAPE include those in the renin-angiotensin system (RAS), NO pathway, and hypoxia-inducible factor pathway (HIF), among others.[5][6] Future genomic testing could provide a much clearer picture of the genetic factors that contribute to HAPE.[5][6]
Pathophysiology

Though it remains a topic of intense investigation, multiple studies and reviews over the last several years have helped to elucidate the proposed mechanism of HAPE. The inciting factor of HAPE is the decrease in partial pressure of arterial oxygen caused by the lower air pressure at high altitudes (pulmonary gas pressures).[1][5][7] The resultant hypoxemia is then thought to precipitate the development of:
- Increased pulmonary arterial and capillary pressures (pulmonary hypertension) secondary to hypoxic pulmonary vasoconstriction.[5][8]
- Increased capillary pressure (hydrostatic pressure) with over-distention of the capillary beds and increased permeability of the vascular endothelium, also known as "stress failure."[5][9] This leads to subsequent leakage of cells and proteins into the alveoli, aka pulmonary edema.[5]
Hypoxic pulmonary vasoconstriction (HPV) occurs diffusely, leading to arterial vasoconstriction in all areas of the lung. This is evidenced by the appearance of "diffuse," "fluffy," and "patchy" infiltrates described on imaging studies of climbers with known HAPE.[5]
Although higher pulmonary arterial pressures are associated with the development of HAPE, the presence of pulmonary hypertension may not in itself be sufficient to explain the development of edema; severe pulmonary hypertension can exist in the absence of clinical HAPE in subjects at high altitude.[5][10]
Diagnosis
The diagnosis of HAPE is purely clinical as many of the symptoms that develop overlap with the clinical presentation of several other diagnoses (differential diagnoses below).[5][2] Before HAPE was understood as a clinical entity it was commonly confused with pneumonia. This fact is what has led to the high mortality rate of HAPE as early recognition and specific treatment are imperative.
HAPE generally develops in the first 2 to 4 days of hiking at altitudes >2,500 meters (8,200 ft), and symptoms seem to worsen most commonly on the second night.[5] Initial signs and symptoms are vague and include dyspnea, decreased exercise capacity, increased recovery time, fatigue, and weakness, especially with walking uphill.[5][2] Patients then develop a dry, persistent cough, and often cyanosis of the lips. Another cardinal feature of HAPE is the rapid progression to dyspnea at rest.[5][2] The development of pink, frothy, or frankly bloody sputum are late features of HAPE.[5][2] In some cases, patients will develop concomitant neurological features such as ataxia, altered consciousness, or frank cerebral edema (High-altitude cerebral edema).[5][2]
| Altitude | SpO2 | PaO2 (mm Hg) |
|---|---|---|
| 1,500 to 3,500 m 4,900 to 11,500 ft |
about 90% | 55-75 |
| 3,500 to 5,500 m 11,500 to 18,000 ft |
75-85% | 40-60 |
| 5,500 to 8,850 m 18,000 to 29,000 ft |
58-75% | 28-40 |
On physical exam, tachypnea, tachycardia, and low-grade fever 38.5o (101.3o F) are common.[5][2] Auscultation of the lungs may reveal rales/crackles in one or both lungs, often starting in the right middle lobe.[5][2] This can be seen on plain radiographs of the chest and CT imaging.[5][2] One very distinct feature of HAPE is that pulse oximetry saturation levels (SpO2) are often markedly decreased from what would be expected for the altitude. Patients typically do not appear as ill as SpO2 and chest X-ray films would suggest.[5][2] The administration of supplemental oxygen rapidly improves clinical symptoms and SpO2 values; in the setting of infiltrative changes on chest X-ray, this is nearly pathognomonic for HAPE.[2]
- Pneumonia
- Bronchitis
- Mucous plugging
- Pulmonary embolism
- Acute coronary syndrome
- Acute decompensated heart failure
- Asthma
- Reactive airway disease
- Exercise-associated hyponatremia
- Pneumothorax
Prevention
As outlined in the 2014 Wilderness Medical Society (WMS) Practice Guidelines on Acute Altitude Illness, the primary recommendation for the prevention of HAPE is gradual ascent.[11] The suggested rate of ascent is the same that applies to the prevention of acute mountain sickness and high-altitude cerebral edema.
The WMS recommends that, above 3,000 metres (9,800 ft), climbers
- not increase the sleeping elevation by more than 500 metres (1,600 ft) a day, and
- include a rest day every 3-4 days (ie, no additional ascent).[11]
In the event that adherence to these recommendations is limited by terrain or logistical factors, the WMS recommends rest days either before or after days with large gains. Overall, WMS recommends that the average ascent rate of the entire trip be less than 500 metres (1,600 ft) per day.[11]
The most studied and preferred medication for pharmacologic prevention of HAPE is nifedipine,[11][2]a pulmonary vasodilator. The recommendation for its use is strongest for individuals with a history of HAPE. According to published data, treatment is most effective if administered one day prior to ascent and continued for four to five days, or until descent below 2,500 meters (8,200 ft).[11][2]
Additional medications that are being considered for prevention but require further research to determine efficacy and treatment guidelines include acetazolamide, salmeterol, tadalafil (and other PDE5 inhibitors), and dexamethasone.[11][2][12] Acetazoladmide has proven to be clinically effective, but formal studies are lacking. Salmeterol is considered an adjunctive therapy to nifedipine, though only in highly susceptible climbers with clearly demonstrated recurrence of HAPE.[11][2] Tadalafil was found to be effective at preventing HAPE in HAPE-s individuals during rapid ascent, but optimal dosing and frequency has yet to be established.[5] Use of dexamethasone is currently indicated for the treatment of moderate-to-severe acute mountain sickness, as well as high-altitude cerebral edema. It has also been found to prevent HAPE[13], but its routine use is not yet recommended.[2][5][11]
Notably, each of these medications acts to block hypoxic pulmonary hypertension, lending evidence to the proposed pathophysiology of HAPE outlined above.[5]
Treatment

Early recognition of HAPE is imperative and recommended first line treatment is descent to a lower altitude as quickly as possible, with symptomatic improvement seen in as few as 500 to 1,000 meters (1,640 feet to 3,281 feet).[1][2][5][14] However, descent is not mandatory and treatment with warming techniques, rest, and supplemental oxygen can rapidly improve symptoms if resources are available (eg, ski resorts).[2][5][11] Oxygen administered at flow rates high enough to maintain an SpO2 at or above 90% is a fair substitute for descent.[2][5][11] In the hospital setting, oxygen is generally administered by nasal cannula or face mask for several hours until the patient is able to maintain oxygen saturations above 90% while breathing ambient air.[2] In remote settings where resources are scarce and descent is not feasible, a reasonable substitute can be the use of a portable hyperbaric chamber, which simulates descent, combined with supplemental oxygen and medications.[2][5][11]
As with prevention, the standard pharmacologic treatment once a climber has developed HAPE is nifedipine,[15] although its use is best in combination with and does not substitute for descent, hyperbaric therapy, or oxygen therapy.[2][5][11] Though they have not formally been studied for the treatment of HAPE, phosphodiesterase type 5 inhibitors such as sildenafil and tadalafil are also effective[13] and can be considered as adjunctive treatment if first-line therapy is not possible; however, they may worsen the headache of mountain sickness.[16] There is no established role for the inhaled beta-agonist salmeterol, though its use can be considered.[2][5][11]
Dexamethasone has a potential role in HAPE, though there are currently no studies to support its effectiveness as treatment.[11] However, as outlined in the 2014 WMS Practice Guidelines, its use is recommended for the treatment of patients with concomitant HAPE and HACE at the treatment doses recommended for HACE alone.[11] Additionally, they support its use in HAPE patients who exhibit neurologic symptoms/hypoxic encephalopathy that cannot be distinguished from HACE.[11]
Epidemiology
| High altitude | 1,500 to 3,500 metres (4,900 to 11,500 ft) |
| Very high altitude | 3,500 to 5,500 metres (11,500 to 18,000 ft) |
| Extreme altitude | 5,500 to 8,850 metres (18,000 to 29,000 ft) |
Incidence of HAPE differs depending on altitude. In general, there is about a 0.2 to 6 percent incidence at 4,500 metres (14,800 ft), and about 2 to 15 percent at 5,500 metres (18,000 ft).[2] It has been reported that about 1 in 10,000 skiers who travel to moderate altitudes in Colorado develop HAPE; one study reported 150 cases over 39 months at a Colorado resort located at 2,928 metres (9,606 ft).[5] About 1 in 50 climbers who ascended Denali [6,194 metres or 20,322 feet] developed pulmonary edema, and as high as 6% of climbers ascending rapidly in the Alps [4,559 metres or 14,957 feet].[5] In climbers who had previously developed HAPE, re-attack rate was up to 60% with ascent to 4,559 metres (14,957 ft) in a 36 hour time period, though this risk was significantly reduced with slower ascent rates.[5]
Research
As noted above, HAPE remains a topic of significant interest and studies that establish more definitive pharmacologic treatment guidelines are needed.
To help understand factors that make some individuals susceptible to HAPE, the International HAPE Database was set up in 2004. The database is administered by APEX, a high altitude medical research charity.[18] Individuals who have previously suffered from HAPE can register with this confidential database to help researchers study the condition.
See also
- Altitude sickness
- Hazards of outdoor recreation
- High-altitude cerebral edema (HACE)
- High-altitude flatus expulsion (HAFE)
References
- ^ a b c Roach, James M.; Schoene, Robert B. (2002). "High-Altitude Pulmonary Edema" (PDF). In Pandolf, Kent B.; Burr, Robert E. (eds.). Medical Aspects of Harsh Environments. Vol. 2. Washington, DC: Borden Institute. pp. 789–814. OCLC 64437370.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Gallagher, MD, Scott A.; Hackett, MD, Peter (August 28, 2018). "High altitude pulmonary edema". UpToDate. Retrieved May 2, 2019.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ "Why do low oxygen levels cause altitude sickness?". Altitude.org.
- ^ "The Lake Louise Consensus on the Definition of Altitude Illness". High Altitude Medicine Guide. Thomas E. Dietz. Retrieved 2012-11-10.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am Auerbach, Paul S. (2017). Wilderness Medicine. Elsevier. pp. 20–25. ISBN 978-0-323-35942-9.
- ^ a b Anamika Gangwar, Subhojit Paul (2015). "High Altitude Pulmonary Edema: An Update on Omics Data and Redefining Susceptibility". Journal of Proteomics & Bioinformatics. 08 (06). doi:10.4172/jpb.1000359.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kenneth Baillie; Alistair Simpson. "Barometric pressure calculator". Apex (Altitude Physiology EXpeditions). Retrieved 2006-08-10.
- ^ Bärtsch, P; Maggiorini, M; Ritter, M; Noti, C; Vock, P; Oelz, O (October 1991). "Prevention of high-altitude pulmonary edema by nifedipine". The New England Journal of Medicine. 325 (18): 1284–9. doi:10.1056/NEJM199110313251805. PMID 1922223.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Swenson, ER; Maggiorini, M; Mongovin, S; et al. (May 2002). "Pathogenesis of high-altitude pulmonary edema: inflammation is not an etiologic factor". JAMA. 287 (17): 2228–35. doi:10.1001/jama.287.17.2228. PMID 11980523.
- ^ Maggiorini, M; Mélot, C; Pierre, S; et al. (April 2001). "High-altitude pulmonary edema is initially caused by an increase in capillary pressure". Circulation. 103 (16): 2078–83. doi:10.1161/01.cir.103.16.2078. PMID 11319198.
- ^ a b c d e f g h i j k l m n o p Luks, MD, Andrew M.; McIntosh, MD, MPH, Scott E.; Grissom, MD, Colin K.; et al. (2014). "Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Acute Altitude Illness: 2014 Update". Wilderness & Environmental Medicine. 25 (24): S4–S14. doi:10.1016/j.wem.2014.06.017. PMID 25498261.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Altitude Diseases - Injuries; Poisoning". Merck Manuals Professional Edition. May 2018. Retrieved 3 August 2018.
- ^ a b Maggiorini, M; Brunner-La Rocca, HP; Peth S; et al. (October 2006). "Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: a randomized trial". Annals of Internal Medicine. 145 (7): 497–506. doi:10.7326/0003-4819-145-7-200610030-00007. PMID 17015867.
- ^ Luks, AM (2008). "Do we have a 'best practice' for treating high altitude pulmonary edema?". High Altitude Medicine & Biology. 9 (2): 111–4. doi:10.1089/ham.2008.1017. PMID 18578641.
- ^ Bärtsch, P; Swenson, Erik R.; Maggiorini, ER; Maggiorini, M (2001). "Update: High altitude pulmonary edema". Advances in Experimental Medicine and Biology. 502: 89–106. doi:10.1007/978-1-4757-3401-0_8. ISBN 978-1-4419-3374-4. PMID 11950158.
- ^ Bates, MG; Thompson, AA; Baillie, JK (March 2007). "Phosphodiesterase type 5 inhibitors in the treatment and prevention of high altitude pulmonary edema". Current Opinion in Investigational Drugs. 8 (3): 226–31. PMID 17408118.
- ^ "Non-Physician Altitude Tutorial". International Society for Mountain Medicine. Archived from the original on 2011-06-06. Retrieved 22 December 2005.
- ^ "International HAPE database". Apex (Altitude Physiology EXpeditions). Retrieved 2006-08-10.
