Staphylococcus epidermidis
| Staphylococcus epidermidis | |
|---|---|
| |
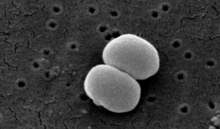
| Scanning electron image of S. epidermidis. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Caryophanales |
| Family: | Staphylococcaceae |
| Genus: | Staphylococcus |
| Species: | S. epidermidis
|
| Binomial name | |
| Staphylococcus epidermidis (Winslow & Winslow 1908)
Evans 1916 | |
| Synonyms | |
|
Staphylococcus albus Rosenbach 1884 | |
Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus.[1] It is part of the normal human microbiota, typically the skin microbiota, and less commonly the mucosal microbiota and also found in marine sponges.[2][3] It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired.[4] S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices.[5] Being part of the normal skin microbiota, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.[6]
Some strains of S. epidermidis are highly salt tolerant and commonly found in marine environment.[3] S.I. Paul et al. (2021)[3] isolated and identified salt tolerant strains of S. epidermidis (strains ISP111A, ISP111B and ISP111C) from Cliona viridis sponges of the Saint Martin's Island Area of the Bay of Bengal, Bangladesh.

Etymology
'Staphylococcus' - bunch of grape-like berries, 'epidermidis' - of the epidermis.[7]
Discovery
Friedrich Julius Rosenbach distinguished S. epidermidis from S. aureus in 1884, initially naming S. epidermidis as S. albus.[8] He chose aureus and albus since the bacteria formed yellow and white colonies, respectively.
Cellular morphology and biochemistry
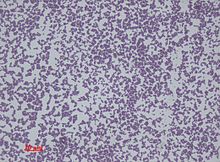
Staphylococcus epidermidis is a very hardy microorganism, consisting of nonmotile, Gram-positive cocci, arranged in grape-like clusters. It forms white, raised, cohesive colonies about 1–2 mm in diameter after overnight incubation, and is not hemolytic on blood agar.[5] It is a catalase-positive,[9] coagulase-negative, facultative anaerobe that can grow by aerobic respiration or by fermentation. Some strains may not ferment.[3][10]
Biochemical tests indicate this microorganism also carries out a weakly positive reaction to the nitrate reductase test. It is positive for urease production, is oxidase negative, and can use glucose, sucrose, and lactose to form acid products. In the presence of lactose, it will also produce gas. Nonpathogenic S. epidermidis unlike pathogenic S. aureus does not possess the gelatinase enzyme, so it cannot hydrolyze gelatin.[11][12] It is sensitive to novobiocin, providing an important test to distinguish it from Staphylococcus saprophyticus, which is coagulase-negative, as well, but novobiocin-resistant.[4]
Similar to those of S. aureus, the cell walls of S. epidermidis have a transferrin-binding protein that helps the organism obtain iron from transferrin. The tetramers of a surface exposed protein, glyceraldehyde-3-phosphate dehydrogenase, are believed to bind to transferrin and remove its iron. Subsequent steps include iron being transferred to surface lipoproteins, then to transport proteins which carry the iron into the cell.[5]
Biochemical characteristics of Staphylococcus epidermidis
Colony, morphological, physiological, and biochemical characteristics of marine S. epidermidis are shown in the table below.[3]
| Test type | Test | Characteristics |
| Colony characters | Size | Pin headed/ Very small |
| Type | Round | |
| Color | Opaque | |
| Shape | Convex | |
| Morphological characters | Shape | Cocci |
| Physiological characters | Motility | – |
| Growth at 6.5% NaCl | + | |
| Biochemical characters | Gram’s staining | + |
| Oxidase | – | |
| Catalase | + | |
| Oxidative-Fermentative | Fermentative | |
| Motility | – | |
| Methyl Red | – | |
| Voges-Proskauer | + | |
| Indole | – | |
| H2S Production | + | |
| Urease | + | |
| Nitrate reductase | + | |
| β-Galactosidase | + | |
| Hydrolysis of | Gelatin | – |
| Aesculin | + | |
| Casein | + | |
| Tween 40 | + | |
| Tween 60 | + | |
| Tween 80 | + | |
| Acid production from | Glycerol | – |
| Galactose | W | |
| D-Glucose | + | |
| D-Fructose | + | |
| D-Mannose | + | |
| Mannitol | – | |
| N-Acetylglucosamine | + | |
| Amygdalin | + | |
| Maltose | + | |
| D-Melibiose | + | |
| D-Trehalose | + | |
| Glycogen | + | |
| D-Turanose | + |
Note: + = Positive, – =Negative, W= Weakly Positive
Virulence and antibiotic resistance
The ability to form biofilms on plastic devices is a major virulence factor for S. epidermidis. One probable cause is surface proteins that bind blood and extracellular matrix proteins. It produces an extracellular material known as polysaccharide intercellular adhesin (PIA), which is made up of sulfated polysaccharides. It allows other bacteria to bind to the already existing biofilm, creating a multilayer biofilm. Such biofilms decrease the metabolic activity of bacteria within them. This decreased metabolism, in combination with impaired diffusion of antibiotics, makes it difficult for antibiotics to effectively clear this type of infection.[5] S. epidermidis strains are often resistant to antibiotics, including rifamycin, fluoroquinolones, gentamicin, tetracycline, clindamycin, and sulfonamides.[13] Methicillin resistance is particularly widespread, with 75-90% of hospital isolates resistance to methicillin.[13] Resistant organisms are most commonly found in the intestine, but organisms living freely on the skin can also become resistant due to routine exposure to antibiotics secreted in sweat.[citation needed]
The Role of Staphylococcus epidermidis in Foot Odor
A common misconception about foot odor and body odor in general is that sweat itself smells and causes people to smell. However, sweat itself is almost entirely odorless. Rather, there are microbes on our skin that metabolize certain compounds in sweat. These microbes are using the body's sweat as a source of food and nutrients and the metabolizing of the compounds is what causes the unpleasant smell [14]. Sweat is also said to be similar to a fingerprint in that everyone has a unique scent from their sweat. Staphylococcus epidermidis is a bacteria that thrives in warm, moist environments and is a common bacteria in the human microbiome[15]. It is this bacteria that is primarily responsible for foot odor because feet have more sweat glands than any other part of the body and thus are often moist, which creates an ideal environment for Staphylococcus epidermidis to live in. The staphylococcus epidermidis has enzymes that degrade the leucine, an essential amino acid, in sweat. This produces unpleasant smelling volatile compounds such as isovaleric acid. Feet with stronger odors have a higher density of microorganisms than those with weaker foot odor[16].
How to Reduce Staphylococcus epidermidis
Completely eliminating Staphylococcus epidermidis on feet is hard to do considering the bacteria is naturally occurring in the human biome. Since this is the case, the best thing to do to eliminate foot odor is to reduce how well the bacteria can live and reproduce on your feet. Cleaning feet thoroughly, especially in places where it is possible for bacteria to accumulate, such as in the crevices between each toe, is a way to reduce the amount of bacteria[17]. If consistent with this, there should be a large reduction in the amount of foot odor one produces. Further, because Staphylococcus epidermidis thrives in moist environments, it is important to dry feet after washing them as to not provide an even more ideal environment for the bacteria to grow. Additionally, it is good to wear breathable socks to help prevent a buildup of moisture on the feet throughout the day[18]. It is also safe to use deodorants or antiperspirants that are used typically to prevent underarm odor on feet. Deodorant masks the smell of foot odor whereas antiperspirants reduce sweating which in turn reduces foot odor[19]. These methods are often successful in reducing odor, however for some people, other factors that affect foot odor such as diet and genetics, may make it more difficult to control the odor. In these cases more involved methods may need to be taken, including botox injections in the feet which last up to three or four months[20].
Disease

As mentioned above, S. epidermidis causes biofilms to grow on plastic devices placed within the body.[13] This occurs most commonly on intravenous catheters and on medical prostheses.[21] Infection can also occur in dialysis patients or anyone with an implanted plastic device that may have been contaminated. It also causes endocarditis, most often in patients with defective heart valves. In some other cases, sepsis can occur in hospital patients. [citation needed]
Antibiotics are largely ineffective in clearing biofilms. The most common treatment for these infections is to remove or replace the infected implant, though in all cases, prevention is ideal. The drug of choice is often vancomycin, to which rifampin or an aminoglycoside can be added.[citation needed] Hand washing has been shown to reduce the spread of infection.
Preliminary research also indicates S. epidermidis is universally found inside affected acne vulgaris pores, where Cutibacterium acnes is normally the sole resident.[22]
The role of Staphylococcus epidermidis in acne vulgaris
Staphylococcus epidermidis in the normal skin is nonpathogenic. But in abnormal lesions, it becomes pathogenic, likely in acne vulgaris. Staphylococcus epidermidis enters the sebaceous gland (colonized by Propionibacterium acnes, the main bacterium that causes acne vulgaris) and damages the hair follicles by producing lipolytic enzymes that change the sebum from fraction to dense (thick) form leading to inflammatory effect.[23]
Moreover, S. epidermidis biofilm formation by releasing the exopolysaccharide intercellular adhesion (PIA) provides the susceptible anaerobic environment to P. acnes colonisation and protects it from the innate human immunity molecules.[24]
Both P. acnes and S. epidermidis can interact to protect the host skin health from pathogens colonisation. But in the case of competition, they use the same carbon source (i.e. glycerol) to produce short chain fatty acids which act as antibacterial agent against each other. Also, S. epidermidis helps in skin homeostasis and reduces the P. acnes pathogenic inflammation by decreasing the TLR2 protein production that induces the skin inflammation.[25]
Identification
The normal practice of detecting S. epidermidis is by using appearance of colonies on selective media, bacterial morphology by light microscopy, catalase and slide coagulase testing. Zobell agar is useful for the isolation of Staphylococcus epidermidis from marine organisms.[3] On the Baird-Parker agar with egg yolk supplement, colonies appear small and black. Increasingly, techniques such as quantitative PCR are being employed for the rapid detection and identification of Staphylococcus strains.[26][27] Normally, sensitivity to desferrioxamine can also be used to distinguish it from most other staphylococci, except in the case of Staphylococcus hominis, which is also sensitive.[28] In this case, the production of acid from trehalose by S. hominis can be used to tell the two species apart.[citation needed]
See also
Notes and references
- ^ Schleifer, K. H.; Kloos, W. E. (1 January 1975). "Isolation and Characterization of Staphylococci from Human Skin I. Amended Descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and Descriptions of Three New Species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus". International Journal of Systematic Bacteriology. 25 (1): 50–61. doi:10.1099/00207713-25-1-50.
- ^ Fey, Paul D; Olson, Michael E (June 2010). "Current concepts in biofilm formation of". Future Microbiology. 5 (6): 917–933. doi:10.2217/fmb.10.56. PMC 2903046. PMID 20521936.
- ^ a b c d e f Paul, Sulav Indra; Rahman, Md. Mahbubur; Salam, Mohammad Abdus; Khan, Md. Arifur Rahman; Islam, Md. Tofazzal (2021-12-15). "Identification of marine sponge-associated bacteria of the Saint Martin's island of the Bay of Bengal emphasizing on the prevention of motile Aeromonas septicemia in Labeo rohita". Aquaculture. 545: 737156. doi:10.1016/j.aquaculture.2021.737156. ISSN 0044-8486.
- ^ a b Levinson, W. (2010). Review of Medical Microbiology and Immunology (11th ed.). pp. 94–99.
- ^ a b c d Salyers, Abigail A. & Whitt, Dixie D. (2002). Bacterial Pathogenesis: A Molecular Approach, 2nd ed. Washington, D.C.: ASM Press. ISBN 978-1-55581-171-6.
- ^ Queck SY, Otto M (2008). "Staphylococcus epidermidis and other Coagulase-Negative Staphylococci". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 978-1-904455-29-5.
- ^ "VetBact".
- ^ Friedrich Julius Rosenbach at Who Named It?
- ^ "Todar's Online Textbook of Bacteriology: Staphylococcus aureus and Staphylococcal Disease". Kenneth Todar, PhD. Retrieved Dec 7, 2013.
- ^ "Bacteria Genomes - STAPHYLOCOCCUS EPIDERMIDIS". Karyn's Genomes. EMBL-EBI. Retrieved December 23, 2011.
- ^ Cruz, Thomas Edison E. dela; Torres, Jeremy Martin O. (2012-11-01). "Gelatin Hydrolysis Test Protocol". www.asmscience.org. Retrieved 2021-01-01.
- ^ Chabi, Roya; Momtaz, Hassan (2019-12-05). "Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran". Tropical Medicine and Health. 47 (1): 56. doi:10.1186/s41182-019-0180-7. ISSN 1349-4147. PMC 6896349. PMID 31844416.
- ^ a b c Otto, Michael (August 2009). "Staphylococcus epidermidis — the 'accidental' pathogen". Nature Reviews Microbiology. 7 (8): 555–567. doi:10.1038/nrmicro2182. PMC 2807625. PMID 19609257.
- ^ "Body Odor: Causes, Changes, Underlying Diseases & Treatment". Cleveland Clinic. Retrieved 2023-05-11.
- ^ Ara, Katsutoshi; Hama, Masakatsu; Akiba, Syunichi; Koike, Kenzo; Okisaka, Koichi; Hagura, Toyoki; Kamiya, Tetsuro; Tomita, Fusao (2006-04-01). "Foot odor due to microbial metabolism and its control". Canadian Journal of Microbiology. 52 (4): 357–364. doi:10.1139/w05-130. ISSN 0008-4166.
- ^ Ara, Katsutoshi; Hama, Masakatsu; Akiba, Syunichi; Koike, Kenzo; Okisaka, Koichi; Hagura, Toyoki; Kamiya, Tetsuro; Tomita, Fusao (2006-04-01). "Foot odor due to microbial metabolism and its control". Canadian Journal of Microbiology. 52 (4): 357–364. doi:10.1139/w05-130. ISSN 0008-4166.
- ^ "How to Get Rid of Smelly Feet: 14 Treatments". Healthline. 2017-07-26. Retrieved 2023-05-11.
- ^ "Smelly feet: Footwear tips, home remedies, and more". www.medicalnewstoday.com. 2017-08-19. Retrieved 2023-05-11.
- ^ "How to Get Rid of Smelly Feet: 14 Treatments". Healthline. 2017-07-26. Retrieved 2023-05-11.
- ^ "Botox for Sweating: How It Works, Cost, Targeted Areas, and Risks". Healthline. 2018-07-18. Retrieved 2023-05-11.
- ^ Hedin, G (1993). "Staphylococcus epidermidis--hospital epidemiology and the detection of methicillin resistance". Scandinavian Journal of Infectious Diseases. Supplementum. 90: 1–59. PMID 8303217.
- ^ Bek-Thomsen, M.; Lomholt, H. B.; Kilian, M. (20 August 2008). "Acne is Not Associated with Yet-Uncultured Bacteria". Journal of Clinical Microbiology. 46 (10): 3355–3360. doi:10.1128/JCM.00799-08. PMC 2566126. PMID 18716234.
- ^ Mustarichie, Resmi; Sulistyaningsih, Sulistiyaningsih; Runadi, Dudi (29 January 2020). "Antibacterial Activity Test of Extracts and Fractions of Cassava Leaves (Manihot esculenta Crantz) against Clinical Isolates of Staphylococcus epidermidis and Propionibacterium acnes Causing Acne". International Journal of Microbiology. 2020: 1975904. doi:10.1155/2020/1975904. PMC 7008253. PMID 32089694.
- ^ Kumar, Bipul; Pathak, Rajiv; Mary, P. Bertin; Jha, Diksha; Sardana, Kabir; Gautam, Hemant K. (1 June 2016). "New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations". Dermatologica Sinica. 34 (2): 67–73. doi:10.1016/j.dsi.2015.12.004.
- ^ Claudel, Jean-Paul; Auffret, Nicole; Leccia, Marie-Thérèse; Poli, Florence; Corvec, Stéphane; Dréno, Brigitte (2019). "Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne?". Dermatology. 235 (4): 287–294. doi:10.1159/000499858. PMID 31112983. S2CID 162170301.
- ^ Francois P, Schrenzel J (2008). "Rapid Diagnosis and Typing of Staphylococcus aureus". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 978-1-904455-29-5.
- ^ Mackay IM (editor). (2007). Real-Time PCR in Microbiology: From Diagnosis to Characterization. Caister Academic Press. ISBN 978-1-904455-18-9.
{{cite book}}:|author=has generic name (help) - ^ Antunes, Ana Lúcia Souza; Secchi, Carina; Reiter, Keli Cristine; Perez, Leandro Reus Rodrigues; Freitas, Ana Lúcia Peixoto De; D'azevedo, Pedro Alves (2008-01-01). "Feasible identification of Staphylococcus epidermidis using desferrioxamine and fosfomycin disks". APMIS. 116 (1): 16–20. doi:10.1111/j.1600-0463.2008.00796.x. PMID 18254775. S2CID 205804740.
Further reading
- Barros, J; Grenho, L; Manuel, CM; Ferreira, C; Melo, L; Nunes, OC; Monteiro, FJ; Ferraz, MP (11 October 2013). "Influence of nanohydroxyapatite surface properties on Staphylococcus epidermidis biofilm formation". Journal of Biomaterials Applications. 28 (9): 1325–1335. doi:10.1177/0885328213507300. hdl:10216/103571. PMID 24122400. S2CID 37361193.
- Dong, Ying; Glaser, Kirsten; Schlegel, Nicolas; Claus, Heike; Speer, Christian P. (November 2019). "An underestimated pathogen: Staphylococcus epidermidis induces pro-inflammatory responses in human alveolar epithelial cells". Cytokine. 123: 154761. doi:10.1016/j.cyto.2019.154761. PMID 31226437. S2CID 195260717.
- Feng, G.; Cheng, Y.; Worobo, R.W.; Borca‐Tasciuc, D.A.; Moraru, C.I. (8 September 2019). "Nanoporous anodic alumina reduces Staphylococcus biofilm formation". Letters in Applied Microbiology. 69 (4): 246–251. doi:10.1111/lam.13201. PMID 31357240.
- Gill, Steven R.; Fouts, Derrick E.; Archer, Gordon L.; Mongodin, Emmanuel F.; DeBoy, Robert T.; Ravel, Jacques; Paulsen, Ian T.; Kolonay, James F.; Brinkac, Lauren; Beanan, Mauren; Dodson, Robert J.; Daugherty, Sean C.; Madupu, Ramana; Angiuoli, Samuel V.; Durkin, A. Scott; Haft, Daniel H.; Vamathevan, Jessica; Khouri, Hoda; Utterback, Terry; Lee, Chris; Dimitrov, George; Jiang, Lingxia; Qin, Haiying; Weidman, Jan; Tran, Kevin; Kang, Kathy; Hance, Ioana R.; Nelson, Karen E.; Fraser, Claire M. (1 April 2005). "Insights on Evolution of Virulence and Resistance from the Complete Genome Analysis of an Early Methicillin-Resistant Staphylococcus aureus Strain and a Biofilm-Producing Methicillin-Resistant Staphylococcus epidermidis Strain". Journal of Bacteriology. 187 (7): 2426–2438. doi:10.1128/JB.187.7.2426-2438.2005. PMC 1065214. PMID 15774886.
- Gotz, Friedrich (March 2002). "Staphylococcus and biofilms". Molecular Microbiology. 43 (6): 1367–1378. doi:10.1046/j.1365-2958.2002.02827.x. PMID 11952892. S2CID 10516046.
- Haidamak, Juciliane; Davila dos Santos, Germana; Lima, Bruna Jacomel Favoreto de Souza; Soares, Valéria Mendes; de Menezes, Raquel Vizzotto; Bisson, Amanda Albino; Talevi, Amanda Santos; Gomes, Renata Rodrigues; Vicente, Vânia Aparecida; Valero, Maria Adela; Klisiowicz, Débora do Rocio (September 2019). "Scalp microbiota alterations in children with pediculosis". Infection, Genetics and Evolution. 73: 322–331. doi:10.1016/j.meegid.2019.05.016. PMID 31121305.
- Izano, Era A.; Amarante, Matthew A.; Kher, William B.; Kaplan, Jeffrey B. (15 January 2008). "Differential Roles of Poly-N-Acetylglucosamine Surface Polysaccharide and Extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis Biofilms". Applied and Environmental Microbiology. 74 (2): 470–476. Bibcode:2008ApEnM..74..470I. doi:10.1128/AEM.02073-07. PMC 2223269. PMID 18039822.
- Méric, Guillaume; Miragaia, Maria; de Been, Mark; Yahara, Koji; Pascoe, Ben; Mageiros, Leonardos; Mikhail, Jane; Harris, Llinos G.; Wilkinson, Thomas S.; Rolo, Joana; Lamble, Sarah; Bray, James E.; Jolley, Keith A.; Hanage, William P.; Bowden, Rory; Maiden, Martin C.J.; Mack, Dietrich; de Lencastre, Hermínia; Feil, Edward J.; Corander, Jukka; Sheppard, Samuel K. (May 2015). "Ecological Overlap and Horizontal Gene Transfer in Staphylococcus aureus and Staphylococcus epidermidis". Genome Biology and Evolution. 7 (5): 1313–1328. doi:10.1093/gbe/evv066. PMC 4453061. PMID 25888688.
- Nakatsuji, Teruaki; Chen, Tiffany H.; Butcher, Anna M.; Trzoss, Lynnie L.; Nam, Sang-Jip; Shirakawa, Karina T.; Zhou, Wei; Oh, Julia; Otto, Michael; Fenical, William; Gallo, Richard L. (28 February 2018). "A commensal strain of Staphylococcus epidermidis protects against skin neoplasia". Science Advances. 4 (2): eaao4502. Bibcode:2018SciA....4.4502N. doi:10.1126/sciadv.aao4502. PMC 5834004. PMID 29507878.
- Otto, Michael (August 2009). "Staphylococcus epidermidis — the 'accidental' pathogen". Nature Reviews Microbiology. 7 (8): 555–567. doi:10.1038/nrmicro2182. PMC 2807625. PMID 19609257.
- Qin, Zhiqiang; Ou, Yuanzhu; Yang, Liang; Zhu, Yuli; Tolker-Nielsen, Tim; Molin, Soeren; Qu, Di (1 July 2007). "Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis". Microbiology. 153 (7): 2083–2092. doi:10.1099/mic.0.2007/006031-0. PMID 17600053.
- Schaeffer, Carolyn R.; Hoang, Tra-My N.; Sudbeck, Craig M.; Alawi, Malik; Tolo, Isaiah E.; Robinson, D. Ashley; Horswill, Alexander R.; Rohde, Holger; Fey, Paul D.; D'Orazio, Sarah E. F. (5 October 2016). "Versatility of Biofilm Matrix Molecules in Staphylococcus epidermidis Clinical Isolates and Importance of Polysaccharide Intercellular Adhesin Expression during High Shear Stress". mSphere. 1 (5). doi:10.1128/mSphere.00165-16. PMC 5064449. PMID 27747298.
- Shahrooei, Mohammad; Hira, Vishal; Khodaparast, Laleh; Khodaparast, Ladan; Stijlemans, Benoit; Kucharíková, Soňa; Burghout, Peter; Hermans, Peter W. M.; Van Eldere, Johan; Camilli, A. (October 2012). "Vaccination with SesC Decreases Staphylococcus epidermidis Biofilm Formation". Infection and Immunity. 80 (10): 3660–3668. doi:10.1128/IAI.00104-12. PMC 3457580. PMID 22802343.
