Fenobam
| |
| Names | |
|---|---|
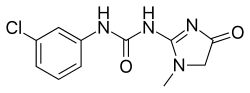
| Preferred IUPAC name
N-(3-Chlorophenyl)-N′-(1-methyl-4-oxo-4,5-dihydro-1H-imidazol-2-yl)urea | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.165.052 |
| MeSH | Fenobam |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H11ClN4O2 | |
| Molar mass | 266.684 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5,[1][2] and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.[3][4][5][6]
Fenobam has anxiolytic effects comparable to those of benzodiazepine drugs,[1][7][8] but was never commercially marketed for the treatment of anxiety due to dose-limiting side effects such as amnesia and psychotomimetic symptoms.[9][10] Following the discovery of its activity as a potent negative allosteric modulator of mGluR5, fenobam has been re-investigated for many applications, with its profile of combined antidepressant, anxiolytic, analgesic and anti-addictive effects potentially useful given the common co-morbidity of these symptoms.[11][12] It has also shown promising initial results in the treatment of fragile X syndrome.[13] It was developed by a team at McNeil Laboratories in the 1970s.[14]
Chemistry
Fenobam is known to exist in five crystalline forms, all of them exhibiting a tautomeric structure with the proton attached to the five membered ring nitrogen. [15]
See also
References
- ^ a b Porter RH; Jaeschke G; Spooren W; et al. (November 2005). "Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity". J. Pharmacol. Exp. Ther. 315 (2): 711–21. doi:10.1124/jpet.105.089839. PMID 16040814. S2CID 386427.
- ^ Marino, MJ; Conn, PJ (2006). "Glutamate-based therapeutic approaches: Allosteric modulators of metabotropic glutamate receptors". Current Opinion in Pharmacology. 6 (1): 98–102. doi:10.1016/j.coph.2005.09.006. PMID 16368268.
- ^ Wållberg, A; Nilsson, K; Osterlund, K; Peterson, A; Elg, S; Raboisson, P; Bauer, U; Hammerland, LG; Mattsson, JP (2006). "Phenyl ureas of creatinine as mGluR5 antagonists. A structure-activity relationship study of fenobam analogues". Bioorganic & Medicinal Chemistry Letters. 16 (5): 1142–5. doi:10.1016/j.bmcl.2005.11.092. PMID 16380255.
- ^ Ceccarelli, SM; Jaeschke, G; Buettelmann, B; Huwyler, J; Kolczewski, S; Peters, JU; Prinssen, E; Porter, R; et al. (2007). "Rational design, synthesis, and structure-activity relationship of benzoxazolones: New potent mglu5 receptor antagonists based on the fenobam structure". Bioorganic & Medicinal Chemistry Letters. 17 (5): 1302–6. doi:10.1016/j.bmcl.2006.12.006. PMID 17189691.
- ^ Jaeschke, G; Porter, R; Büttelmann, B; Ceccarelli, SM; Guba, W; Kuhn, B; Kolczewski, S; Huwyler, J; et al. (2007). "Synthesis and biological evaluation of fenobam analogs as mGlu5 receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 17 (5): 1307–11. doi:10.1016/j.bmcl.2006.12.033. PMID 17196387.
- ^ Gichinga, Moses G.; Olson, Jeremy P.; Butala, Elizabeth; Navarro, Hernán A.; Gilmour, Brian P.; Mascarella, S. Wayne; Carroll, F. Ivy (2011). "Synthesis and Evaluation of Metabotropic Glutamate Receptor Subtype 5 Antagonists Based on Fenobam". ACS Medicinal Chemistry Letters. 2 (12): 882–884. doi:10.1021/ml200162f. PMC 3328804. PMID 22523618.
- ^ Pecknold, JC; McClure, DJ; Appeltauer, L; Wrzesinski, L; Allan, T (1982). "Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study". Journal of Clinical Psychopharmacology. 2 (2): 129–33. doi:10.1097/00004714-198204000-00010. PMID 7042771.
- ^ Goldberg, ME; Salama, AI; Patel, JB; Malick, JB (1983). "Novel non-benzodiazepine anxiolytics". Neuropharmacology. 22 (12B): 1499–504. doi:10.1016/0028-3908(83)90118-1. PMID 6142427. S2CID 44419672.
- ^ Palucha, A; Pilc, A (2007). "Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs". Pharmacology & Therapeutics. 115 (1): 116–47. doi:10.1016/j.pharmthera.2007.04.007. PMID 17582504.
- ^ Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E, Malyshkin A, Greco S, Barberi C, Danysz W (May 2009). "The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning". Neuropharmacology. 57 (2): 97–108. doi:10.1016/j.neuropharm.2009.04.011. PMID 19426746. S2CID 207224547.
- ^ Carroll, FI (2008). "Antagonists at metabotropic glutamate receptor subtype 5: Structure activity relationships and therapeutic potential for addiction". Annals of the New York Academy of Sciences. 1141: 221–32. doi:10.1196/annals.1441.015. PMID 18991960. S2CID 45268931.
- ^ Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RW (June 2009). "The mGlu5 antagonist fenobam is analgesic and has improved in vivo selectivity as compared to the prototypical antagonist MPEP". The Journal of Pharmacology and Experimental Therapeutics. 330 (3): 834–43. doi:10.1124/jpet.109.154138. PMC 2729799. PMID 19515968.
- ^ Berry-Kravis, E; Hessl, D; Coffey, S; Hervey, C; Schneider, A; Yuhas, J; Hutchison, J; Snape, M; et al. (2009). "A pilot open label, single dose trial of fenobam in adults with fragile X syndrome". Journal of Medical Genetics. 46 (4): 266–71. doi:10.1136/jmg.2008.063701. PMC 2658751. PMID 19126569.
- ^ US Patent 3983135 4-Oxo-2-imidazolidinylidene ureas
- ^ Thomas, Sajesh P. (2012). "Polymorphism and tautomeric preference in fenobam and the utility of NLO response to detect polymorphic impurities". Chemical Communications. 48 (85): 10559–10561. doi:10.1039/C2CC34912D. PMID 23000909.
