Iodomethane

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodomethane
| |||
| Other names
Monoiodomethane, Methyl iodine, MeI, Halon 10001, UN 2644
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.000.745 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH3I | |||
| Molar mass | 141.94 g/mol | ||
| Appearance | Clear colourless liquid with acrid odor | ||
| Density | 2.28 g/cm3 (20 °C)[1] | ||
| Melting point | −66.45 °C (−87.61 °F; 206.70 K) | ||
| Boiling point | 42.43 °C (108.37 °F; 315.58 K) | ||
| 1.4 g/100 mL (20 °C) | |||
| log P | 1.51 | ||
| Vapor pressure | 50 kPa at 20 °C 53.32 at 25.3 °C 166.1 kPa at 55 °C | ||
Refractive index (nD)
|
1.531 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Explosive limits | 8.5 - 66% | ||
Threshold limit value (TLV)
|
2 ppm (TWA), 5 ppm (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
0.78 mmol/kg (mouse, s.c.)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. This dense volatile liquid is related to methane by replacement of one hydrogen atom by an atom of iodine. Methyl iodide is miscible with organic solvents and is colourless. It is naturally emitted by rice plantations in small amounts.[2] Methyl iodide is used in organic synthesis to deliver a methyl group, and was approved for use as a pesticide in a controversial decision by the EPA in 2007.[3]
Chemical properties
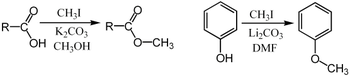
Methyl iodide is an excellent substrate for SN2 substitution reactions. It is sterically open for attack by nucleophiles, and iodide is a good leaving group. For example, it can be used for the methylation of phenols or carboxylic acids:[4]
In these examples, the base (K2CO3 or Li2CO3) removes the acidic proton to form the carboxylate or phenoxide anion, which serves as the nucleophile in the SN2 substitution.
Iodide is a "soft" anion which means that methylation with MeI tends to occur at the "softer" end of an ambidentate nucleophile. For example, reaction with thiocyanate ion favours attack at Template:Sulfur rather than "hard" Template:Nitrogen, leading mainly to methyl thiocyanate (CH3SCN) rather than CH3NCS. This behavior is relevant to the methylation of stabilized enolates such as those derived from 1,3-dicarbonyl compounds. Methylation of these and related enolates can occur on the harder oxygen atom or the (usually desired) carbon atom. With methyl iodide, C-alkylation nearly always predominates.
MeI is also an important precursor to methylmagnesium iodide or "MeMgI", which is a common reagent. Because MeMgI forms readily, it is often prepared in instructional laboratories as an illustration of Grignard reagents. The use of MeMgI has been somewhat superseded by the commercially available methyl lithium.
In the Monsanto process, MeI forms in situ from the reaction of methanol and hydrogen iodide. The CH3I then reacts with carbon monoxide in the presence of a rhodium complex to form acetyl iodide, the precursor to acetic acid after hydrolysis. Most acetic acid is prepared by this method.
MeI can also be used to prepare dimethylmercury, by reacting 2 moles of MeI with a 2/1-molar sodium amalgam (2 moles of sodium, 1 mol of mercury).
MeI hydrolyzes at 270 °C forming hydrogen iodide, carbon monoxide and carbon dioxide.
Preparation
Methyl iodide is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus.[5] The iodinating reagent is phosphorus triiodide that is formed in situ:
Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of calcium carbonate:[5]
- (CH3O)2SO2 + KI → CH3I + CH3OSO2OK
The CH3I can be purified by distillation followed by washing with Na2S2O3 to remove iodine.
Methyl iodide can also be prepared by the reaction of methanol with potassium iodide, catalyzed by acid:
- CH3OH + KI + H2SO4 → CH3I + K2SO4 + H2O
The reaction is carried out at low temperature and the water generated in the reaction is trapped by excess sulfuric acid so the reaction is not reversible. The generated methyl iodide can be distilled from the reaction mixture.
Uses
Use as a methylating agent
Methyl iodide is an excellent reagent for methylation, but there are some disadvantages to its use. It has a high equivalent weight: one mole of methyl iodide weighs almost three times as much as one mole of methyl chloride. However, the chloride is a gas (as is methyl bromide), making it more awkward to work with than methyl iodide, which is a liquid. Methyl chloride is a poorer methylating reagent than methyl iodide, though it is often adequate.
Iodides are generally expensive relative to the more common chlorides and bromides, though methyl iodide is reasonably affordable; on a commercial scale the toxic dimethyl sulfate is preferred, since it is both cheap and liquid. The iodide leaving group in methyl iodide may cause side reactions, as it is a powerful nucleophile. Finally, being highly reactive, methyl iodide is more dangerous for laboratory workers than related chlorides and bromides. When considering alternatives to methyl iodide, it is necessary to consider cost, handling, risk, chemical selectivity, and ease of reaction work-up.
Use as a pesticide
Methyl Iodide has also been proposed for use as a fungicide, herbicide, insecticide, nematicide, and as a soil disinfectant, replacing bromomethane (banned under the Montreal Protocol). In a controversial October 2007 decision, the United States Environmental Protection Agency, under the Bush Administration, approved the use of methyl iodide as a soil fumigant in some cases, although it cannot yet be used in Washington and New York due to lack of state approval.[6][7]
On December 1, 2010, methyl iodide use was approved as a pesticide in the State of California.[8] Following the approval, a coalition of environmentalists, researchers and farmers -- including California State Assemblyman Bill Monning, D-Carmel -- gathered at locations in Santa Cruz County and six other sites around California to protest the impending use of methyl iodide and to ask governor-elect Jerry Brown to block it. Critics of methyl iodide cite both the cancer-causing aspects of the pesticide and the potential for it to leach out of fields, contaminating water supplies.[9]Objections were also raised by two dozen California legislators and 54 scientists, including five Nobel laureates[10]
UC Davis Chemistry professor, Dr. Neil Schore, testified at an Informational Hearing on Methyl Iodide in the California State Legislature in late 2009 and made the following statement regarding the use of methyl iodide as a soil fumigant in California:
Since methyl iodide is almost entirely going to percolate downward as a liquid, it's going to inevitably mix with ground water and the only chemistry that's going to take place is ultimate reaction with the ground water to form methyl alcohol . . . this conversion to methyl alcohol, which is, as we all know, wood alcohol, two teaspoons and you're blind, half a cup and you're dead. This is going to contaminate groundwater and since methyl iodide boils at a lower temperature than water does, even distillation will not remove it. So think MTBE if you will, in terms of groundwater contamination. I don't know if these aspects have been studied, these, again, are just basic chemical facts. [11]
Toxicity and biological effects
Methyl iodide has an LD50 for oral administration to rats 76 mg/kg, and in the liver it undergoes rapid conversion to S-methylglutathione.[12] It is a possible carcinogen based on ACGIH or NTP classification, but not according to the Environmental Protection Agency.Methyl Iodide (Iodomethane) However, methyl iodide is included on the California Prop.65 list of chemicals known by the state to cause cancer. [13]
Breathing methyl iodide fumes can cause lung, liver, kidney and central nervous system damage. It causes nausea, dizziness, coughing and vomiting. Prolonged contact with skin causes burns. Massive inhalation causes pulmonary edema.
References
- ^ a b Merck Index, 11th Edition, 6002
- ^ K. R. Redeker, N.-Y. Wang, J. C. Low, A. McMillan, S. C. Tyler, and R. J. Cicerone (2000). "Emissions of Methyl Halides and Methane from Rice Paddies". Science. 290 (5493): 966–969. doi:10.1126/science.290.5493.966. PMID 11062125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zitto, Kelly "Methyl iodide gains state OK for use on crops". San Francisco Chronicle. December 2, 2010.
- ^ Avila-Zárraga, J. G., Martínez, R. (2001). "Efficient methylation of carboxylic acids with potassium hydroxide/methyl sulfoxide and iodomethane". Synthetic Communications. 31 (14): 2177–2183. doi:10.1081/SCC-100104469.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b King, C. S.; Hartman, W. W. (1943). "Methyl Iodide". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 2, p. 399. - ^ "EPA approves new pesticide despite scientists' concerns". Los Angeles Times. October 6, 2007.
- ^ "California sun and spray". High Country News. August 4, 2009.
- ^ "Calif approves use of pesticide linked to cancer". San Francisco Chronicle. December 1, 2010.
- ^ "Statewide protest targets new strawberry pesticide". San Jose Mercury News. November 30, 2010.
- ^ Zitto, Kelly "Methyl iodide gains state OK for use on crops". San Francisco Chronicle. December 2, 2010.
- ^ Dr. Neil Schore: testimony Assembly Labor and Employment Committee. Citizennewscaster.com. December 8, 2009.
- ^ Johnson, M. K. (1966). "Metabolism of iodomethane in the rat". Biochem. J. 98: 38–43.
- ^ http://www.oehha.ca.gov/prop65/prop65_list/Newlist.html
Additional sources
- March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2
- Sulikowski, G. A.; Sulikowski, M. M. (1999). in Coates, R.M.; Denmark, S. E. (Eds.) Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation New York: Wiley, pp. 423–26.
- Bolt H. M., Gansewendt B. (1993). "Mechanisms of carcinogenicity of methyl halides". Crit Rev Toxicol. 23 (3): 237–53. doi:10.3109/10408449309105011. PMID 8260067.
External links
- International Chemical Safety Card 0509
- NIOSH Pocket Guide to Chemical Hazards. "#0420". National Institute for Occupational Safety and Health (NIOSH).
- IARC Summaries & Evaluations: Vol. 15 (1977), Vol. 41 (1986), Vol. 71 (1999)
- Metabolism of iodomethane in the rat
- Iodomethane NMR spectra
- Jones, Nicola (24 September 2009). "Strawberry pesticide leaves sour taste: Methyl iodide use by Californian farmers up for review". Nature News. Retrieved 25 September 2009.