Carbyne
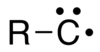
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methine | |
| Systematic IUPAC name
Methylidyne | |
| Other names
λ1-Carbane
Carbon(I) hydride | |
| Identifiers | |
3D model (JSmol)
|
|
| 7801830 | |
| ChemSpider | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In chemistry, a carbyne is a monovalent carbon radical species containing an electrically neutral univalent carbon atom with three non-bonded electrons.[1]
Gas phase/reactive intermediate
A carbyne can occur as a short-lived reactive intermediate. For instance, fluoromethylidyne (CF) can be detected in the gas phase by spectroscopy as an intermediate in the flash photolysis of CHFBr2.[2] The carbon atom was generally found to be an electronic doublet: the valence electrons are arranged as one radical (unpaired electron) and one electron pair, leaving a vacant atomic orbital, rather than being a tri-radical. The carbon atom is a complex hybridization, so the simple Hund's rule analysis of a simple atom containing 3 p orbitals (or 4 sp3 hybrids) is not correct.
Organometallic ligand
Carbynes are incorporated in metal carbyne complexes[3][4] as a trivalent ligand. For example, in [WBr(CO)2(2,2'-bipyridine)C-Aryl] and [WBr(CO)2(PPh3)2C-NR2]. An example of how to make such a compound would be to react [W(CO)6] with Lithium diisopropylamide to form [(iPr2N)(OLi)C=W(CO)5]. This is then reacted with either oxalyl bromide or triphenylphosphine dibromide followed by triphenyl phosphine. Another method is to treat a methoxy metal carbene with a lewis acid.[5]
Polymeric form/carbon allotrope
Carbyne, is also another name for Linear Acetylenic Carbon[6] (LAC), an allotrope of carbon that has the chemical structure[7] -(C≡C)n- . Carbon in this modification is linear with sp orbital hybridisation, and is a polymer with alternating single and triple bonds. This type of carbyne is of considerable interest to nanotechnology as its Young's modulus is forty times that of diamond, the hardest known material.[8] The existence of carbyne itself as a carbon allotrope is controversial, as the properties and synthetic methods seem consistent with generation of fullerenes.[9] However, polyalkyne oligomers called polyynes as substructures of larger compounds are well known and actively researched as a substitute.[10] Carbyne chains of over 300 carbons have been prepared and appear to be reasonably stable as long as the terminal alkynes on the chain are capped rather than having a free acetylenic H atom.[11] The analysis in this study specifically demonstrated that the result was a carbyne-like structure rather than a fullerene.
An analysis of a synthesized linear carbon allotrope found it to have a cumulene electronic structure—sequential double bonds along an sp-hybridized carbon chain—rather than the alternating triple–single pattern of linear carbyne.[12]
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carbynes". doi:10.1351/goldbook.C00854
- ^ "Chemistry of carbynes: reaction of CF, CCl, and CBr with alkenes". J. Am. Chem. Soc. 105: 2489–2490. 1983. doi:10.1021/ja00346a072.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Furno, F.; Fox, T.; Berke, H. trans-W(CMes)(dmpe)2H: a W+ H– ion pair (PDF). EURO-HYDRIDES 2000.
{{cite conference}}: External link in|conferenceurl=|conferenceurl=ignored (|conference-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Details of their reactivity and that of the related carbenes is shown at http://www.thieme-chemistry.com/thieme-chemistry/sos/info/include/pdf/sc02.pdf.
- ^ Jaeger, M.; Stumpf, R.; Troll, C.; Fischer, H. (2000). "Novel hepta-coordinated molybdenum(II) and tungsten(II) carbene complexes by oxidative decarbonylation of Mo(0) and W(0) carbene complexes". Chem. Commun.: 931–932. doi:10.1039/B002228O.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Dangerously Seeking Linear Carbon". Science. 312: 1009–1110. 2006. doi:10.1126/science.1125999.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Carbyne and Carbynoid Structures. Physics and Chemistry of Materials with Low-Dimensional Structures. Vol. 21. 1999. p. 452. ISBN 0-7923-5323-4.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ "Harder than Diamond: Determining the Cross-Sectional Area and Young's Modulus of Molecular Rods". Angew. Chem. Int. Ed. 44: 7432–7435. 2005. doi:10.1002/ange.200502448.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Kroto, H. (2010). "Carbyne and other myths about carbon". RSC Chemistry World.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Synthesis of extended polyynes: Toward carbyne". Comptes Rendus Chimie. 12 (3): 341–358. 2009. doi:10.1016/j.crci.2008.10.004.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Synthesis of Linear Acetylenic Carbon: The "sp" Carbon Allotrope". Science. 267 (5196): 362–367. 1995. doi:10.1126/science.267.5196.362.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Linear carbon allotrope – carbon atom wires prepared by pyrolysis of starch". Chemical Physics Letters. 385 (5–6): 477–480. 2004. doi:10.1016/j.cplett.2004.01.007.
{{cite journal}}: Unknown parameter|authors=ignored (help)
See also
- Tobe, Y.; Wakabayashi, T. "Chapter 9. Carbon-Rich Compounds: Acetylene-Based Carobn Allotropes". In Diederich, F.; Stang, P. J.; Tykwinski, R. R. (ed.). Acetylene Chemistry Acetylene chemistry: chemistry, biology, and material science. pp. 387–426.
{{cite book}}: Check|url=value (help); Cite has empty unknown parameter:|1=(help)CS1 maint: multiple names: authors list (link)

