User:Ichthyovenator/sandbox
Eurypterid
| Ichthyovenator/sandbox | |
|---|---|

| |
| Fossil specimen of Eurypterus remipes housed at the State Museum of Natural History Karlsruhe in Karlsruhe, Germany. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Clade: | Sclerophorata |
| Order: | †Eurypterida Burmeister, 1843 |
| Suborders | |
| |
| Synonyms | |
| |
Eurypterids, often informally referred to as sea scorpions, are an extinct group of arthropods that form the order Eurypterida. The earliest known eurypterids date to the Darriwilian stage of the Ordovician period 467.3 million years ago, though the group is likely to have first appeared either during the Early Ordovician or Late Cambrian period. The Eurypterida is the most diverse Paleozoic chelicerate order in terms of species, containing approximately 250 valid species. Following their appearance during the Ordovician, eurypterids would become major components of marine faunas during the Silurian, from which a majority of eurypterid species have been described. The Silurian genus Eurypterus accounts for more than 90% of all known eurypterid specimens. Though the group continued to diversify during the subsequent Devonian period, the eurypterids were heavily affected by the Late Devonian extinction event and would decline in number and diversity until they became extinct during the Permian–Triassic extinction event (or sometime shortly before) 251.902 million years ago.
Although popularly called "sea scorpions", only the earliest eurypterids were marine, with many later forms living in brackish or fresh water, and they were not true scorpions. Some studies suggest that a dual respiratory system was present, which would have allowed for short periods of time in terrestrial environments. The name Eurypterida comes from the Ancient Greek words εὐρύς (eurús), meaning "broad" or "wide", and πτερόν (pteron), meaning "wing", referring to the pair of wide swimming appendages present in many members of the group.
The eurypterids include the largest known arthopods to have ever lived. The largest, such as Jaekelopterus, reached 2.5 meters (8.2 ft) in length. Eurypterids were not uniformly large and most species were less than 20 centimeters (7.9 in) long; the smallest eurypterids, Alkenopterus and Eocarcinosoma, were only 3 centimeters (1.2 in) long. Eurypterid fossils have been recovered from every continent, though a vast majority of fossils are from fossil sites in North America and Europe due to the group primarily having lived in the waters around and within the ancient supercontinent of Euramerica. Only a handful of eurypterid groups spread beyond the confines of Euramerica and a few genera, such as Adelophthalmus and Pterygotus, achieved a cosmopolitan distribution with fossils being found worldwide.
Morphology
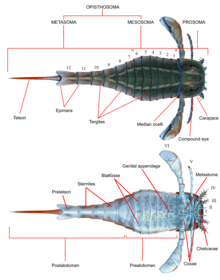
Like all other arthropods, eurypterids possessed segmented bodies and jointed appendages (limbs) covered in a cuticle composed of proteins and chitin. As in other chelicerates, the body was divided into two tagmata (sections); the frontal prosoma (head) and posterior opisthosoma (abdomen).[1] The prosoma was covered by a carapace (sometimes referred to as the "prosomal shield") on which the compound eyes and ocelli (simple eye-like sensory organs) were located.[2]
The prosoma also bore six pairs of appendages which are usually referred to as appendage pairs I to VI by eurypterid researchers. The first pair of appendages, the only pair placed before the mouth, are referred to as the chelicerae (differently developed, but the same organs as the fangs of spiders) and were equipped with small pincers used to manipulate food fragments and push them into the mouth.[2] In one lineage, the Pterygotidae, the chelicerae were large and long, with strong, well-developed teeth on specialised chelae (claws).[3] The subsequent pairs of appendages, numbers II to VI, possessed gnathobases (or "tooth-plates") on the coxae (limb segments) used for feeding. These appendages were generally walking legs that were cylindrical in shape and were covered in spines in some species. In most lineages, the limbs tended to get larger the further back they were. In the Eurypterina suborder, the larger of the two eurypterid suborders, the sixth pair of appendages was also modified into a swimming paddle to aid in traversing aquatic environments.[2]
The opisthosoma compromised 12 segments and the telson, the very posteriormost segment, which in most species took the form of a blade-like shape.[2] In some lineages, notably the Pterygotioidea and the Hibbertopteridae, the telson was flattened and may possibly have been used as a rudder while swimming. Some genera within the Carcinosomatoidea superfamily, notably Eusarcana, had a telson similar to that of modern scorpions and may have been capable of using it to inject venom.[4][5] The coxae of the sixth pair of appendages were overlaid by a plate that is referred to as the metastoma, originally derived from a complete exoskeleton segment. The opisthosoma itself can be divided either into a "mesosoma" (compromising segments 1 to 6) and "metasoma" (compromising segments 7 to 12) or into a "preabdomen" (compromising segments 1 to 7) and "postabdomen" (compromising segments 8 to 12).[2]
The underside of the opisthosoma was covered in structures evolved from modified opisthosomal appendages. Throughout the opisthosoma, these structures formed plate-like structures termed blatfüsse (German for "leaf-feet"). These create a branchial (relating to gills) chamber between preceding blatfüsse and the ventral surface of the opisthosoma itself, which contained the respiratory organs. The second to sixth opisthosomal segments also contained oval organs that have been interpreted as organs that aid in respiration. These organs, termed kiemenplatten, or "gill tracts", would potentially have aided eurypterids to breath air above water whilst blatfüssen, similar to organs in modern horseshoe crabs, would cover the parts that serve for underwater respiration.[2]
The opisthosomal appendages of segments 7 and 8 (that is the first and second segments of the opisthosoma) were fused into a structure termed the genital operculum, occupying most of the underside of the eighth opisthosomal segment. Near the anterior margin of this structure, the genital appendage (also referred to as the zipfel or the median abdominal appendage) protruded. This appendage, often preserved very prominently, has consistently been interpreted as part of the reproductive system and occurs in two recognized types, assumed to correspond to male and female.[2]
Biology
Size

Eurypterids were highly variable in size, depending on factors such as lifestyle, living environment and taxonomic affinity. Smaller eurypterids were likely formidable predators just like their larger relatives and sizes around 100 centimeters (3.3 ft) are common in most eurypterid groups.[6] The smallest eurypterids, Alkenopterus burglahrensis and Eocarcinosoma batrachophthalmus, measured just 3 centimeters (1.2 in) in length whilst the largest exceeded 2 meters (6.6 ft).[7]
The largest eurypterid, and the largest known arthropod to have ever lived, is Jaekelopterus rhenaniae. A chelicera from the Emsian Klerf Formation of Willwerath, Germany measured 36.4 centimeters (14.3 in) in length, but is missing a quarter of its length, suggesting that the full chelicera would have been 45.5 centimeters (17.9 in) long. If the proportions between body length and chelicerae match those of its closest relatives, where the ratio between claw size and body length is relatively consistent, the specimen of Jaekelopterus that possessed the chelicera in question would have measured between 233 and 259 centimeters (7.64 and 8.50 ft), average 2.5 meters (8.2 ft), in length. With the chelicerae extended, another meter would be added to this length. This estimate exceeds the maximum body size of all other known giant arthropods by almost half a meter even if the extended chelicerae are not included.[8]
The family of Jaekelopterus, the Pterygotidae, is noted for several unusually large species. Both Acutiramus, the largest species A. bohemicus measuring 2.1 meters (6.9 ft), and Pterygotus, the largest species P. grandidentatus measuring 1.75 meters (5.7 ft), were gigantic.[8] Several different contributing factors to the large size of the pterygotids have been suggested, including courtship behaviour, predation and competition over environmental resources.[9]
Giant eurypterids were not limited to the Pterygotidae family. An isolated 12.7 centimeters (5.0 in) long fossil metastoma of the carcinosomatoid eurypterid Carcinosoma punctatum indicates that the animal would have reached a length of 2.2 meters (7.2 ft) in life, rivalling the pterygotids in size.[10] Another giant was Pentecopterus decorahensis, a primitive carcinosomatoid, which is estimated to have reached lengths of 1.7 meters (5.6 ft).[11]
Typical of large eurypterids is a lightweight build. Factors such as locomotion, energy costs in moulting and respiration as well as the actual physical properties of the exoskeleton limits the size that arthropods can reach. A lightweight construction significantly decreases the influence of these factors. Pterygotids were particularly lightweight, with most fossilized large body segments preserving as thin and unmineralized.[8] Lightweight adaptations are present in other giant paleozoic arthropods as well, such as the giant insect Arthropleura, and are possibly vital for the evolution of giant size in arthropods.[8][12]
In addition to the lightweight giant eurypterids, some deep-bodied forms in the family Hibbertopteridae were also very large. A carapace from the Carboniferous of Scotland referred to the species Hibbertoperus scouleri measures 65 cm wide. As Hibbertopterus was very wide compared to its length, the animal in question could possibly have measured just short of 2 meters (6.6 ft) in length. More robust than the pterygotids, this giant Hibbertopterus would possibly have rivalled the largest pterygotids in weight, if not surpassed them, and as such be among the heaviest arthropods.[13]
Locomotion
The two eurypterid suborders, Eurypterina and Stylonurina, are primarily separated by the morphology of their final pair of appendages. In the Stylonurina, this appendage takes the form of a long and slender walking leg whilst in the Eurypterina, the leg is modified and broadened into a swimming paddle.[14] The legs other than the swimming paddle of many eurypterines were far too small to do much more than allow them to crawl across the sea floor. In contrast, a number of Stylonurines had elongated and powerful legs that might even have allowed them to walk even on land (similar to modern crabs).[15]
A fossil trackway discovered in Carboniferous-aged fossil deposits of Scotland in 2005, attributed to the stylonurine eurypterid Hibbertopterus due to a matching size (the trackmaker was estimated to have been about 1.6 meters (5.2 ft) long) and inferred leg anatomy, is the largest terrestrial trackway (measuring 6 meters (20 ft) long and averaging 95 centimeters (3.12 ft) in width) made by an arthropod found thus far and the first record of land locomotion by an eurypterid. The trackway provides evidence for that some eurypterids could survive in terrestrial environments, at least for short periods of time, and also reveals information about the stylonurine gait. In Hibbertopterus, as in most eurypterids, the pairs of appendages are all different in size (referred to as a heteropodous limb condition). These differently sized pairs would have moved in phase and the short stride length indicates that Hibbertopterus would have crawled with an exceptionally slow speed, at least on land. The large telson was dragged along the ground and left a large central groove behind the animal. Slopes in the tracks at random intervals suggest that the motion was jerky.[16] The gait of smaller stylonurines, such as Parastylonurus, would probably have been faster and more precise.[17]
The functionality of the eurypterine swimming paddles varied from group to group. In the Eurypteroidea, the paddles were similar in shape to oars and the condition of the joints in their appendages ensured that their paddles could only be moved in near-horizontal planes; not upwards or downwards. Some other groups, such as the Pterygotioidea, would not have possessed this condition and would probably have been able to swim faster.[18] Most eurypterines are generally agreed to have utilized a rowing type of propulsion similar to that of crabs and water beetles. Larger individuals may have been capable of underwater flying (or subaqueous flight) in which the motion and shape of the paddles are enough to generate lift, similar to the swimming of sea turtles and sea lions. This type of movement has a relatively slower acceleration rate than the rowing type, especially since adults have proportionally smaller paddles than juveniles. However, since the larger sizes of adults mean a higher drag coefficient, using this type of propulsion is more energy-efficient.[19]
Some eurypterines, such as Mixopterus (as inferred from attributed fossil trackways), were not necessarily good swimmers. Mixopterus likely kept mostly to the bottom, using its swimming paddles for occasional bursts of movements vertically, with the fourth and fifth pairs of appendages positioned backwards to produce minor movement forwards. While walking, it would probably have used a gait similar to that of most modern insects. The weight of its long abdomen would have been balanced by two heavy and specialized frontal appendages and the center of gravity might have been adjustable by raising and positioning the tail.[20]
Feeding

No fossil gut contents are known from eurypterids, and as such direct evidence for their diet is lacking. Most, if not all, eurypterids are thought to have been carnivorous. The eurypterid biology is particularly suggestive of a carnivorous lifestyle. Not only were many large (in general, most predators tend to be larger than their prey) but they also had stereoscopic vision.[21] The legs of many eurypterids were covered in thin spines, used both for locomotion and for the gathering of food. In some groups, these spiny appendages became heavily specialized. In some eurypterids in the Carcinosomatoidea, forward-facing appendages were large in size and possessed enormously elongated spines (as in Mixopterus and Megalograptus). In derived members of the Pterygotioidea, the appendages were instead completely without spines but with specialized claws instead.[22] Eurypterids lacking these specialized appendages likely fed in a manner similar to modern horseshoe crabs, by grabbing and shredding food with their appendages before pushing it into their mouth using their chelicerae.[23]
Fossils preserving digestive tracts have been reported from fossils of various eurypterids, among them Carcinosoma, Acutiramus and Eurypterus. Though a potential anal opening has been reported from a specimen of Buffalopterus, it is more likely that the anus was opened through the thin cuticle between the last segment before the telson and the telson itself, as in modern horseshoe crabs.[21]
A coprolite discovered in deposits of Ordovician age in Ohio containing fragments of a trilobite and eurypterid Megalograptus ohioensis in association with full specimens of the same eurypterid species have been suggested to represent evidence of cannibalism. Structures found in Silurian deposits of Scotland, interpreted as eurypterid coprolites from the species Lanarkopterus dolichoschelus, contain fragments of agnathan fish and fragments of smaller specimens of Lanarkopterus itself, further reinforcing the idea that some eurypterids may have been cannibalistic.[21]
Reproductive biology
As in many other entirely extinct groups, understanding and researching the reproduction and sexual dimorphism of eurypterids is difficult, as they are only known from fossilized shells and carapaces. In some cases, there might not be enough apparent differences to differentiate different sexes based on morphology alone.[15] Sometimes two sexes of the same species have been interpreted as two different species, as was the case with two species of Drepanopterus (D. bembycoides and D. lobatus).[24]
The eurypterid prosoma is made up of the first six exoskeleton segments fused together into a larger structure. The seventh segment (thus the first opisthosomal segment) is referred to as the metastoma and the eighth segment (distinctly plate-like) is called the operculum and contains the genital aperature. The underside of this segment is occupied by the genital operculum, a structure originally evolved from ancestral seventh and eighth pair of appendages. In its center, as in modern horseshoe crabs, is a genital appendage. This appendage, an elongated rod with an internal duct, is found in two distinct morphs, generally referred to as "type A" and "type B".[15] These genital appendages are often preserved prominently in fossils and have been the subject of various different interpretations of eurypterid reproduction and sexual dimorphism.[25]
Type A appendages are in general longer than type B appendages. In some genera they are divided into different numbers of sections, such as in Eurypterus where the type A appendage is divided into three but the type B appendage only into two.[26] Such division of the genital appendage is common in eurypterids, but the number is not universal, for instance the appendages of both types in the Pterygotidae family are completely undivided.[27] The type A appendage is also armed with two curved spines referred to as furca (Latin for "fork"). The presence of furca in the type B appendage is also possible and the structure may represent the unfused tips of the appendages. Located in between the dorsal and ventral surfaces of the blatfuss associated with the type A appendages are a set of organs traditionally described as either "tubular organs" or "horn organs". These organs are most often interpreted as spermathecae (organs for storing sperm), though this function is yet to be conclusively proven.[28] In arthropods, spermathecae are used to store the spermatophore received from males, which would imply that the type A appendage is the female morph and the type B appendage is the male.[15] Further evidence for the type A appendages representing the female morph of genital appendages comes in their more complex construction (a general trend for female arthropod genitalia). It is possible that the greater length of the type A appendage means that it was used as an ovipositor (used to deposit eggs).[29] The different types of genital appendages are not necessarily the only feature that distinguishes between the sexes of eurypterids. Depending on the genus and species in question other features such as size, the amount of ornamentation and the proportional width of the body can be the result of sexual dimorphism.[2]
The primary function of the long, assumed female, type A appendages was likely to take up spermatophore from the substrate into the reproductive tract rather than to serve as an ovipositor as arthropod ovipositors are generally much longer than eurypterid type A appendages. By rotating the sides of the operculum, it would have been possible to lower the appendage from the body. Due to the way different plates overlay at its location, the appendage would have been impossible to move without muscular contractions moving around the operculum and would thus have been kept in place whenever not used. The furca on the type A appendages may have aided in breaking open the spermatophore to release the free sperm inside for uptake. The "horn organs", possibly spermathecae, are thought to have been connected directly to the appendage via tracts but these supposed tracts remain unpreserved in available fossil material.[30]
Type B appendages, assumed male, would have produced, stored and perhaps shaped spermatophore in a heart-shaped structure on the dorsal surface of the appendage. A broad genital opening would have allowed large amounts of spermatophore to be released at once. The long furca associated with type B appendages, perhaps capable of being lowered like the type A appendage, could have been used to detect whether or not a substrate would have been suitable for spermatophore deposition.[31]
Evolutionary history
Origins

Until 1882 no eurypterids were known from before the Silurian. Discoveries throughout the twentieth century and modern times have expanded the knowledge of early eurypterids from the Ordovician period.[32] The earliest eurypterids known today, the megalograptid Pentecopterus, date from the Darriwilian stage Middle Ordovician, 467.3 million years ago.[33] There are also reports of even earlier fossil eurypterids in deposits of Late Tremadocian (Early Ordovician) age in Morocco, but these have yet to be thoroughly studied.[34]
Pentecopterus was a relatively derived eurypterid, part of the megalograptid family within the carcinosomatoid superfamily. Its derived position suggests that most eurypterid clades, at least within the eurypterine suborder, had already been established at this point during the Middle Ordovician.[35] The earliest known stylonurine eurypterid, Brachyopterus,[6] is also Middle Ordovician in age and the presence of members of both suborders indicates that primitive stem-eurypterids would have preceded them, though these are so far unknown in the fossil record. The presence of several euryterid clades during the Middle Ordovician suggests that eurypterids either originated during the Early Ordovician and experienced a rapid and explosive radiation and diversification soon after the first forms evolved, or that the group originated much earlier, perhaps during the Cambrian period.[35]
As such, the exact eurypterid time of origin remains unknown. Though fossils referred to as "primitive eurypterids" have occasionally been described from deposits of Cambrian or even Precambrian age,[36] these fossils are not recognized as eurypterids, and sometimes not even as related forms, today. Some animals previously seen as primitive eurypterids, such as the genus Strabops from the Cambrian of Missouri,[37] are now classified as aglaspidids or strabopids. The aglaspidids, once seen as primitive chelicerates, are now seen as a group more closely related to trilobites.[38]
The fossil record of Ordovician eurypterids is quite poor. The majority of eurypterids once reportedly known from the Ordovician have since proven to be misidentifications or pseudofossils and today, only 11 species can be confidently identified as representing Ordovician eurypterids. These taxa fall into two distinct ecological categories; large and active predators from the ancient continent of Laurentia and demersal and basal animals from the continents Avalonia and Gondwana.[33] The Laurentian predators, classified in the family Megalograptidae (compromising the genera Echinognathus, Megalograptus and Pentecopterus), are likely to represent the first truly successful eurypterid group, experiencing a small radiation during the Late Ordovician.[39]
Silurian
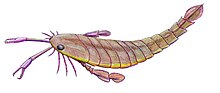
Eurypterids were most diverse and abundant between the Middle Silurian and the Early Devonian, with an absolute peak in diversity during the Pridoli epoch, 423 to 419.2 million years ago, of the very latest Silurian.[14] This peak in diversity has been recognized since the early twentieth century; of the approximately 150 species of eurypterids known in 1916, more than half were from the Silurian and a third were from the Late Silurian alone.[32]
Though stylonurine eurypterids generally remained rare and low in number, as had been the case during the preceding Ordovician, eurypterine eurypterids experienced a rapid rise in diversity and number.[40] In most Silurian fossil beds, eurypterine eurypterids account for 90 % of all eurypterids present.[41] Though some were likely already present by the Late Ordovician (simply missing from the fossil record so far),[35] a vast majority of eurypterid groups are first recorded in strata of Silurian age. These include both stylonurine groups such as the Stylonuroidea, Kokomopteroidea and Mycteropoidea as well as eurypterine groups such as the Pterygotioidea, Eurypteroidea and Waeringopteroidea.[42]
The most successful eurypterid by far was the Middle to Late Silurian Eurypterus, a generalist, equally likely to engage in predation or scavenging. Thought to mainly hunt small and soft-bodied invertebrates, such as worms,[43] species of the genus (of which the most common is the type species, E. remipes) account for more than 90 % (perhaps as many as 95 %) of all known fossil eurypterid specimens.[41] Despite their vast number, Eurypterus are only known from a relatively short temporal range, first appearing during the Late Llandovery epoch (around 432 million years ago) and being extinct by the end of the Pridoli epoch.[44] Eurypterus was also restricted to the minor supercontinent Euramerica (composed of the equatorial continents Avalonia, Baltica and Laurentia), which had been completely colonized by the genus during its merging, and was unable to cross the vast expanses of ocean separating this continent from other parts of the world, such as the southern supercontinent Gondwana. As such, Eurypterus was limited geographically to the coastlines and shallow inland seas of Euramerica.[41][45]
Appearing during the Late Silurian were the pterygotid eurypterids, large and specialized forms with several new adaptations, such as large and flattened telsons capable to be used as rudders and large and specialized chelicerae with enlarged pincers for handling (and potentially in some cases killing) prey.[3][4] Though the largest members of the family appeared in the Devonian, large 2+ meter (6.5+ ft) pterygotids such as Acutiramus were already present during the Late Silurian.[7] Their ecology ranged from generalized predatory behavior to ambush predation and some, such as Pterygotus itself, were active apex predators in Late Silurian marine ecosystems.[46] The pterygotids were also evidently capable of crossing oceans, becoming one of only two eurypterid groups to achieve a cosmopolitan distribution.[47]
Devonian

Though the eurypterids continued to be abundant and diversify during the Early Devonian (for instance leading to the evolution of the pterygotid Jaekelopterus, the largest of all arthropods), the group was one of many heavily affected by the Late Devonian extinction. The extinction event, only known to affect marine life (particularly trilobites, brachiopods and reef-building organisms) effectively crippled the abundance and diversity previously seen within the eurypterids.[48]
A major decline in diversity had already begun during the Early Devonian and eurypterids were rare in marine environments by the Late Devonian. The group saw elevated extinction rates during the Frasnian (extinction of four families) and Famennian (extinction of five families) stages.[48] The decline occured chiefly in marine groups, which primarily impacted the eurypterine eurypterids. Only one group of stylonurines (the family Parastylonuridae) went extinct in the Early Devonian whilst only two families of eurypterines survived into the Late Devonian at all (families Adelophthalmidae and Waeringopteridae). The eurypterines experienced their most major declines in the Early Devonian, during which over 50 % of their diversity was lost in just 10 million years. Stylonurines on the other hand persisted through the period with more or less consistent diversity and abundance but were affected during the Frasnian and Famennian, in which many of the older groups were replaced by new forms in the families Mycteroptidae and Hibbertopteridae.[49]
It is possible that the catastrophic extinction patterns seen in the eurypterine suborder are related to the emergance of more derived fish. Eurypterine decline begins at the point when agnathan fish are beginning to get more developed and coincides with the emergence of placoderms (armored fish) in both North America and Europe.[50] Only three eurypterid families; Adelophthalmidae, Hibbertopteridae and Mycteroptidae (whose fossil record at this time only covers the Late Carboniferous), survived the extinction event in its entirety. All of these were compromised entirely of freshwater animals, rendering the eurypterids extinct in marine environments.[48] With marine eurypterid predators gone, sarcopterygian fish, such as the rhizodonts, were the new apex predators in marine environments.[50]
Stylonurines of the surviving hibbertopterid and mycteroptid families completely avoided competition with fish by evolving towards a new and distinct ecological niche. The Hibbertopteridae experienced a radiation and diversification through the Late Devonian and Early Carboniferous, the last ever radiation within the eurypterids, which gave rise to several new forms capable of "sweep-feeding" (raking through the substrate in search of prey).[51]
Carboniferous and Permian

Through the Carboniferous, stylonurines continued to be represented by hibbertopterids and mycteroptids. The sole surviving eurypterine family, Adelophthalmidae, was represented only by a single genus, Adelophthalmus. The hibbertopterids and Adelophthalmus would survive into the Permian.[52]
Adelophthalmus became the most common of all late Paleozoic eurypterids, existeing in greater number and diversity than surviving stylonurines, and diversified in the absence of other eurypterines.[53] Out of the 31 species referred to Adelophthalmus, 22 (70 %) are from the Carboniferous alone.[54] The genus reached its peak diversity in the Late Carboniferous. Though Adelophthalmus had already been relatively widespread and represented on all major landmasses in the Late Devonian, the amalgamation of Pangaea into a global supercontinent over the course of the last two periods of the Paleozoic allowed Adelophthalmus to gain an almost worldwide distribution.[41]
During the Late Carboniferous and Early Permian Adelophthalmus was widespread, living primarily in brackish and freshwater environments adjacent to coastal plains. These environments were maintained by favorable climate conditions that did not persist as climate changes owing to Pangaea's formation altered depositional and vegetational patterns across the world. With their habitat gone, Adelophthalmus dwindled in number and went extinct in the Leonardian stage of the Early Permian.[55]
Hibbertopterids continued to survive for some time, with two genera known from Permian strata; Hastimima and Campylocephalus.[56] Hastimima went extinct during the Early Permian,[57] as Adelophthalmus had, while Campylocephalus persisted longer. A massive incomplete carapace from Late Permian (Changhsingian stage) deposits in Russia represents the sole fossil remains of the species C. permianus, which might have reached 1.4 meters (4.6 ft) in length.[7] This giant was the last known surviving eurypterid.[6] No eurypterids are known from fossil beds higher than the Permian, indicating that the last eurypterids died either in the catastrophic extinction event at its end or at some point shortly before it. This extinction event, the Permian-Triassic extinction event, is the most devastating mass extinction event recorded and also rendered many other Paleozoic groups, such as the trilobites, extinct.[58]
History of study
Text
Classification
Text
Stylonurina - Lamsdell et al. 2010
Eurypterina (minus "Megalograptoidea") - Tetlie 2007
| Eurypterida | |
References
Citations
- ^ Størmer 1955, p. 23.
- ^ a b c d e f g h Braddy & Dunlop 1997, pp. 437–439.
- ^ a b Tetlie & Briggs 2009, p. 1141.
- ^ a b Plotnick & Baumiller 1988, p. 22.
- ^ Clarke & Ruedemann 1912, p. 244.
- ^ a b c Tetlie 2007, p. 557.
- ^ a b c Lamsdell & Braddy 2009, Supplementary information.
- ^ a b c d Braddy, Poschmann & Tetlie 2008, p. 107.
- ^ Briggs 1985, pp. 157–158.
- ^ Kjellesvig-Waering 1961, p. 830.
- ^ Lamsdell et al. 2015, p. 15.
- ^ Kraus & Brauckmann 2003, pp. 5–50.
- ^ Tetlie 2008, p. 19.
- ^ a b Tetlie 2007, p. 559.
- ^ a b c d Palaeos.
- ^ Whyte 2005, p. 576.
- ^ Selden 1999, p. 43.
- ^ Selden 1999, p. 45.
- ^ Selden 1999, pp. 44–46.
- ^ Hanken & Størmer 1975, pp. 262–267.
- ^ a b c Selden 1999, p. 46.
- ^ Selden 1999, p. 47.
- ^ Hembree, Platt & Smith 2014, p. 77.
- ^ Lamsdell, Braddy & Tetlie 2009, p. 1119.
- ^ Braddy & Dunlop 1997, p. 436.
- ^ Braddy & Dunlop 1997, p. 438.
- ^ Braddy, Poschmann & Tetlie 2008, p. 108.
- ^ Braddy & Dunlop 1997, p. 439.
- ^ Braddy & Dunlop 1997, p. 449.
- ^ Braddy & Dunlop 1997, pp. 450–452.
- ^ Braddy & Dunlop 1997, pp. 454–455.
- ^ a b O'Connell 1916, p. 11.
- ^ a b Lamsdell et al. 2015, p. 1.
- ^ Van Roy, Briggs & Gaines 2015, p. 6.
- ^ a b c Lamsdell et al. 2015, p. 29.
- ^ O'Connell 1916, p. 12.
- ^ O'Connell 1916, p. 13.
- ^ Ortega‐Hernández, Legg & Braddy 2012, p. 15.
- ^ Tetlie 2007, p. 569.
- ^ Tetlie 2007, p. 567.
- ^ a b c d Tetlie 2007, p. 570.
- ^ Dunlop, Penney & Jekel 2018, pp. 17–30.
- ^ Selden 1999, p. 44.
- ^ Tetlie 2006, p. 410.
- ^ Tetlie & Rábano 2007, p. 124.
- ^ McCoy et al. 2015, p. 3.
- ^ Tetlie 2007, p. 571.
- ^ a b c Hallam & Wignall 1997, p. 70.
- ^ Lamsdell & Braddy 2009, p. 265.
- ^ a b Lamsdell & Braddy 2009, p. 266.
- ^ Lamsdell & Braddy 2009, p. 268.
- ^ Dunlop, Penney & Jekel 2018, pp. 19 & 24.
- ^ Tetlie & Van Roy 2006, p. 79.
- ^ Dunlop, Penney & Jekel 2018, p. 24.
- ^ Kues & Kietzke 1981, p. 727.
- ^ Dunlop, Penney & Jekel 2018, p. 19.
- ^ White 1927, p. 575.
- ^ Bergstrom & Dugatkin 2012, p. 515.
Bibliography
- Bergstrom, Carl T.; Dugatkin, Lee Alan (2012). Evolution. Norton. ISBN 978-0393913415.
{{cite book}}: CS1 maint: ref duplicates default (link) - Braddy, Simon J.; Dunlop, Jason A. (1997). "The functional morphology of mating in the Silurian eurypterid, Baltoeurypterus tetragonophthalmus (Fischer, 1839)". Zoological Journal of the Linnean Society. 120 (4): 435–461. doi:10.1111/j.1096-3642.1997.tb01282.x. ISSN 0024-4082.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Braddy, Simon J.; Poschmann, Markus; Tetlie, O. Erik (2008). "Giant claw reveals the largest ever arthropod". Biology Letters. 4 (1): 106–109. doi:10.1098/rsbl.2007.0491. PMC 2412931. PMID 18029297.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Briggs, Derek E. G. (1985). "Gigantism in Palaeozoic arthropods". Special Papers in Palaeontology. 33: 157–158.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Clarke, John Mason; Ruedemann, Rudolf (1912). The Eurypterida of New York. University of California Libraries. ISBN 978-1125460221.
{{cite book}}: CS1 maint: ref duplicates default (link) - Dunlop, Jason A.; Penney, David; Jekel, Denise (2018). "A summary list of fossil spiders and their relatives". World Spider Catalog (PDF). Natural History Museum Bern.
{{cite book}}: CS1 maint: ref duplicates default (link) - Hallam, Anthony; Wignall, Paul B. (1997). Mass Extinctions and Their Aftermath. Oxford University Press. ISBN 978-0198549161.
{{cite book}}: CS1 maint: ref duplicates default (link) - Hanken, Nils-Martin; Størmer, Leif (1975). "The trail of a large Silurian eurypterid" (PDF). Fossils and Strata. 4: 255–270.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Hembree, Daniel I.; Platt, Brian F.; Smith, Jon J. (2014). Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Springer Science & Business. ISBN 978-9401787208.
{{cite book}}: CS1 maint: ref duplicates default (link) - Kjellesvig-Waering, Erik N. (1961). "The Silurian Eurypterida of the Welsh Borderland". Journal of Paleontology. 35 (4): 789–835. JSTOR 1301214.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Kraus, Otto; Brauckmann, Carsten (2003). "Fossil giants and surviving dwarfs. Arthropleurida and Pselaphognatha (Atelocerata, Diplopoda): characters, phylogenetic relationships and construction". Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg. 40: 5–50.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Kues, Barry S.; Kietzke, Kenneth K. (1981). "A Large Assemblage of a New Eurypterid from the Red Tanks Member, Madera Formation (Late Pennsylvanian-Early Permian) of New Mexico". Journal of Paleontology. 55 (4): 709–729.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Lamsdell, James C.; Braddy, Simon J. (2009). "Cope's Rule and Romer's theory: patterns of diversity and gigantism in eurypterids and Palaeozoic vertebrates". Biology Letters. 6 (2): 265–269. doi:10.1098/rsbl.2009.0700. PMC 2865068. PMID 19828493.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Lamsdell, James C.; Braddy, Simon J.; Tetlie, O. Erik (2009). "Redescription of Drepanopterus abonensis (Chelicerata: Eurypterida: Stylonurina) from the late Devonian of Portishead, UK". Palaeontology. 52 (5): 1113–1139. doi:10.1111/j.1475-4983.2009.00902.x. ISSN 1475-4983.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Lamsdell, James C.; Briggs, Derek E. G.; Liu, Huaibao; Witzke, Brian J.; McKay, Robert M. (2015). "The oldest described eurypterid: a giant Middle Ordovician (Darriwilian) megalograptid from the Winneshiek Lagerstätte of Iowa". BMC Evolutionary Biology. 15 (169). doi:10.1186/s12862-015-0443-9. PMC 4556007. PMID 26324341.
{{cite journal}}: CS1 maint: ref duplicates default (link) CS1 maint: unflagged free DOI (link) - McCoy, Victoria E.; Lamsdell, James C.; Poschmann, Markus; Anderson, Ross P.; Briggs, Derek E. G. (2015). "All the better to see you with: eyes and claws reveal the evolution of divergent ecological roles in giant pterygotid eurypterids". Biology Letters. 11 (8). doi:10.1098/rsbl.2015.0564. PMC 4571687. PMID 26289442.
{{cite journal}}: CS1 maint: ref duplicates default (link) - O'Connell, Marjorie (1916). "The Habitat of the Eurypterida". The Bulletin of the Buffalo Society of Natural Sciences. 11 (3): 1–278.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Ortega‐Hernández, Javier; Legg, David A.; Braddy, Simon J. (2012). "The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda". Cladistics. 29: 15–45. doi:10.1111/j.1096-0031.2012.00413.x. ISSN 1502-3931.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Plotnick, Roy E.; Baumiller, Tomasz K. (1988). "The pterygotid telson as a biological rudder". Lethaia. 21 (1): 13–27. doi:10.1111/j.1502-3931.1988.tb01746.x. ISSN 1502-3931.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Selden, Paul (1999). "Autecology of Silurian Eurypterids" (PDF). Special Papers in Palaeontology. 32: 39–54. ISSN 0038-6804. Archived from the original (PDF) on August 3, 2011.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help)CS1 maint: ref duplicates default (link) - Størmer, Leif (1955). "Merostomata". Treatise on Invertebrate Paleontology, Part P Arthropoda 2, Chelicerata. University of Kansas Press. ASIN B0043KRIVC.
{{cite book}}: CS1 maint: ref duplicates default (link) - Tetlie, O. Erik (2006). "Two new Silurian species of Eurypterus (Chelicerata: Eurypterida) from Norway and Canada and the phylogeny of the genus" (PDF). Journal of Systematic Palaeontology. 4 (4): 397–412. doi:10.1017/S1477201906001921. ISSN 1478-0941.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Tetlie, O. Erik; Van Roy, Peter (2006). "A reappraisal of Eurypterus dumonti Stainier, 1917 and its position within the Adelophthalmidae Tollerton, 1989" (PDF). Bulletin de L'Institut Royal Des Sciences Naturelles De Belgique. 76: 79–90.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Tetlie, O. Erik (2007). "Distribution and dispersal history of Eurypterida (Chelicerata)". Palaeogeography, Palaeoclimatology, Palaeoecology. 252 (3–4): 557–574. doi:10.1016/j.palaeo.2007.05.011. ISSN 0031-0182.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Tetlie, O. Erik; Rábano, Isabel (2007). "Specimens of Eurypterus (Chelicerata, Eurypterida) in the collections of Museo Geominero (Geological Survey of Spain), Madrid" (PDF). Boletín Geológico y Minero. 118 (1): 117–126. ISSN 0366-0176. Archived from the original (PDF) on July 22, 2011.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help)CS1 maint: ref duplicates default (link) - Tetlie, O. Erik (2008). "Hallipterus excelsior, a Stylonurid (Chelicerata: Eurypterida) from the Late Devonian Catskill Delta Complex, and Its Phylogenetic Position in the Hardieopteridae". Bulletin of the Peabody Museum of Natural History. 49 (1): 19–30. doi:10.3374/0079-032X(2008)49[19:HEASCE]2.0.CO;2.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Tetlie, O. Erik; Briggs, Derek E. G. (2009). "The origin of pterygotid eurypterids (Chelicerata: Eurypterida)". Palaeontology. 52 (5): 1141–1148. doi:10.1111/j.1475-4983.2009.00907.x. ISSN 0024-4082.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Van Roy, Peter; Briggs, Derek E. G.; Gaines, Robert R. (2015). "The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician". Journal of the Geological Society. 172. doi:10.1144/jgs2015-017. ISSN 0016-7649.
{{cite journal}}: CS1 maint: ref duplicates default (link) - White, David (1927). "Flora of the Hermit Shale, Grand Canyon, Arizona". Proceedings of the National Academy of Sciences of the United States of America. 13 (8): 574–575. PMC 1085121.
{{cite journal}}: CS1 maint: ref duplicates default (link) - Whyte, Martin A. (2005). "A gigantic fossil arthropod trackway". Nature. 438: 576. doi:10.1038/438576a.
{{cite journal}}: CS1 maint: ref duplicates default (link)
Websites
- M. Alan, Kazlev (2002). "Palaeos - Eurypterida". www.palaeos.com. Archived from the original on 13 August 2007.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help)