Thyroid
| thyroid | |
|---|---|
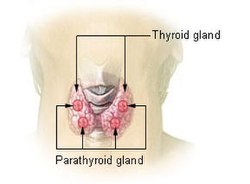 Thyroid and parathyroid. | |
| Details | |
| Precursor | Thyroid diverticulum (an extension of endoderm into 2nd Branchial arch) |
| System | Endocrine system |
| Identifiers | |
| Latin | glandula thyroidea |
| MeSH | D013961 |
| TA98 | A11.3.00.001 |
| TA2 | 3863 |
| FMA | 9603 |
| Anatomical terminology | |
In vertebrate anatomy, the thyroid gland or simply, the thyroid, is one of the largest endocrine glands in the body, and is not to be confused with the "parathyroid glands" (a completely different set of glands). The thyroid gland is found in the neck, inferior to (below) the thyroid cartilage (also known as the 'Adam's Apple') and at approximately the same level as the cricoid cartilage. The thyroid controls how quickly the body uses energy, makes proteins, and controls how sensitive the body should be to other hormones.
The thyroid gland participates in these processes by producing thyroid hormones, the principal ones being triiodothyronine (T3) and thyroxine (T4). These hormones regulate the rate of metabolism and affect the growth and rate of function of many other systems in the body. T3 and T4 are synthesized utilizing both iodine and tyrosine. The thyroid gland also produces a hormone called 'calcitonin', which plays a role in calcium homeostasis.
The thyroid gland is controlled by the hypothalamus and pituitary (to be specific, the anterior pituitary). The thyroid gland gets its name from the Greek word for "shield", after the shape of the related thyroid cartilage. The most common problems of the thyroid gland consist of an over-active thyroid gland, referred to as 'hyperthyroidism', and an under-active thyroid gland, referred to as 'hypothyroidism'.
Anatomy
The thyroid gland is a butterfly-shape organ and is composed of two cone-like lobes or wings, lobus dexter (right lobe) and lobus sinister (left lobe), connected via the isthmus. The organ is situated on the anterior side of the neck, lying against and around the larynx and trachea, reaching posteriorly the oesophagus and carotid sheath. It starts cranially at the oblique line on the thyroid cartilage (just below the laryngeal prominence, or 'Adam's Apple'), and extends inferiorly to approximately the fifth or sixth tracheal ring.[1] It is difficult to demarcate the gland's upper and lower border with vertebral levels because it moves position in relation to these during swallowing.
The thyroid gland is covered by a fibrous sheath, the capsula glandulae thyroidea, composed of an internal and external layer. The external layer is anteriorly continuous with the lamina pretrachealis fasciae cervicalis and posteriorolaterally continuous with the carotid sheath. The gland is covered anteriorly with infrahyoid muscles and laterally with the sternocleidomastoid muscle also known as sternomastoid muscle. On the posterior side, the gland is fixed to the cricoid and tracheal cartilage and cricopharyngeus muscle by a thickening of the fascia to form the posterior suspensory ligament of Berry.[2][3] The thyroid gland's firm attachment to the underlying trachea is the reason behind its movement with swallowing.[4] In variable extent, Lalouette's Pyramid, a pyramidal extension of the thyroid lobe, is present at the most anterior side of the lobe. In this region, the recurrent laryngeal nerve and the inferior thyroid artery pass next to or in the ligament and tubercle.
Between the two layers of the capsule and on the posterior side of the lobes, there are on each side two parathyroid glands.
The thyroid isthmus is variable in presence and size, and can encompass a cranially extending pyramid lobe (lobus pyramidalis or processus pyramidalis), remnant of the thyroglossal duct. The thyroid is one of the larger endocrine glands, weighing 2-3 grams in neonates and 18-60 grams in adults, and is increased in pregnancy.
The thyroid is supplied with arterial blood from the superior thyroid artery, a branch of the external carotid artery, and the inferior thyroid artery, a branch of the thyrocervical trunk, and sometimes by the thyroid ima artery, branching directly from the brachiocephalic trunk. The venous blood is drained via superior thyroid veins, draining in the internal jugular vein, and via inferior thyroid veins, draining via the plexus thyroideus impar in the left brachiocephalic vein.
Lymphatic drainage passes frequently the lateral deep cervical lymph nodes and the pre- and parathracheal lymph nodes. The gland is supplied by parasympathetic nerve input from the superior laryngeal nerve and the recurrent laryngeal nerve.
Thyroid Gland Evolution
Thyroid cells phylogenetically derived from primitive iodide-concentrating gastroenteric cells (endostyle) which, during evolution, migrated and specialized in uptake and storage of iodine in follicular cellular structures, also in order to adapt the organisms from iodine-rich sea to iodine-deficient land. Venturi et al.[5]suggested that iodide has an ancestral antioxidant function in all iodide-concentrating cells from primitive Algae to more recent Vertebrates. In 2008, this ancestral antioxidant action of iodides has been experimentally confirmed by Küpper et al.[6]. Since 700 million years ago thyroxine is present in fibrous exoskeletal scleroproteins of the lowest invertebrates (Porifera and Anthozoa), without showing any hormonal action. When some primitive marine Chordates started to emerge from the iodine-rich sea and transferred to iodine-deficient fresh water and finally land, their diet became iodine deficient. Therefore, during progressive slow adaptation to terrestrial life, the primitive vertebrates learned to use the primitive thyroxine in order to transport antioxidant iodide into the cells. So, the remaining triiodothyronine (T3), the real active hormone, became active in the metamorphosis and thermogenesis for a better adaptation of the organisms to terrestrial environment ( fresh water, atmosphere, gravity, temperature and diet ). In fact, the U.S. Food and Nutrition Board and Institute of Medicine recommended daily allowance of iodine ranges from 150 micrograms /day for adult humans to 290 micrograms /day for lactating mothers. However, the thyroid gland needs no more than 70 micrograms /day to synthesize the requisite daily amounts of T4 and T3. These higher recommended daily allowance levels of iodine seem necessary for optimal function of a number of body systems, including lactating breast, gastric mucosa, salivary glands, oral mucosa, thymus, epidermis, choroid plexus and brain[7], etc.[8][9][10].
See: Iodine in biology
Embryological development
In the fetus, at 3–4 weeks of gestation, the thyroid gland appears as an epithelial proliferation in the floor of the pharynx at the base of the tongue between the tuberculum impar and the copula linguae at a point latter indicated by the foramen cecum. The thyroid then descends in front of the pharyngeal gut as a bilobed diverticulum through the thyroglossal duct. Over the next few weeks, it migrates to the base of the neck. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct.
Thyrotropin-releasing hormone (TRH) and thyroid-stimulating hormone (TSH) start being secreted from the fetal hypothalamus and pituitary at 18-20 weeks of gestation, and fetal production of thyroxine (T4) reach a clinically significant level at 18–20 weeks.[11] Fetal triiodothyronine (T3) remains low (less than 15 ng/dL) until 30 weeks of gestation, and increases to 50 ng/dL at term.[11] Fetal self-sufficiency of thyroid hormones protects the fetus against e.g. brain development abnormalities caused by maternal hypothyroidism.[12] However, preterm births can suffer neurodevelopmental disorders due to lack of maternal thyroid hormones due their own thyroid being insufficiently developed to meet their postnatal needs.[13]
The portion of the thyroid containing the parafollicular C cells, those responsible for the production of calcitonin, are derived from the 4th pharyngeal pouch endoderm. This is first seen as the ultimobranchial body, which joins the primordial thyroid gland during its descent to its final location in the anterior neck.
Histology
At the microscopic level, there are three primary features of the thyroid:[14]
| Feature | Description |
| Follicles | The thyroid is composed of spherical follicles that selectively absorb iodine (as iodide ions, I-) from the blood for production of thyroid hormones. Twenty-five percent of all the body's iodide ions are in the thyroid gland. Inside the follicles, colloid serve as a reservoir of materials for thyroid hormone production and, to a lesser extent, act as a reservoir for the hormones themselves. Colloid is rich in a protein called thyroglobulin. |
| Thyroid epithelial cells (or "follicular cells") |
The follicles are surrounded by a single layer of thyroid epithelial cells, which secrete T3 and T4. When the gland is not secreting T3/T4 (inactive), the epithelial cells range from low columnar to cuboidal cells. When active, the epithelial cells become tall columnar cells. |
| Parafollicular cells (or "C cells") |
Scattered among follicular cells and in spaces between the spherical follicles are another type of thyroid cell, parafollicular cells, which secrete calcitonin. |
Disorders
Disorders of the thyroid gland fall into the following categories:
Hyperthyroidism
Hyperthyroidism is an overactive thyroid. It is the overproduction of the "thyroid hormones" (T3 and T4) by the thyroid gland to which hyperthyroidism refers. The most common cause of hyperthyroidism is a disease called "Graves' Disease". Graves' Disease is a 'diffuse toxic goiter' in which the thyroid gland enlarges as a result of the thyroid glands' overproduction of the T3 & T4 hormones.
Graves' disease is considered to be an autoimmune disease and is the most common cause of thyroid gland overactivity (hyperthyroidism). Graves' disease is much more common in women than in men. Graves' Disease results from excess stimulation of the thyroid gland and usually presents with symptoms in the 2-3rd decade of life. Symptoms include: An enlarged thyroid (goitre), protruding eyes (exopthalmos), palpitations, excess sweating, diarrhea, weight loss, muscle weakness and unusual sensitivity to heat. One treatment of Grave's disease involves the patient taking an oral dose of radioactive iodine, resulting in permanent destruction of cells in the thyroid, thus rendering them permanently inactive. The patient may then be treated with daily replacement hormone therapy as a result of a new found hypothyroidism. Another option may be surgery in which the thyroid gland is partially/fully removed.
Hypothyroidism
Hypothyroidism is the underproduction of "thyroid hormones" (T3 and T4). Hypothyroid disorders occur when the thyroid gland is inactive or underactive as a result of improper formation from birth, or the removal in whole or the removal in part of the thyroid gland.
Symptoms include: abnormal weight gain, tiredness, baldness, temperature intolerance (both heat and cold), and palpitation.
Initial hyperthyroidism followed by hypothyroidism
This is the overproduction of T3 and T4 followed by the underproduction of T3 and T4. There are two types: "Hashimoto's Thyroiditis" and "Postpartum Thyroiditis".
Hashimoto's thyroiditis is an autoimmune disorder whereby the body's own immune system reacts with the thyroid tissues. At the beginning, the gland is overactive, and then becomes underactive as the gland is destroyed resulting in too little thyroid hormone production or hypothyroidism. Hashimoto's is most common in middle-age females and tend to run in families. Also more common in individuals with hashimoto's thyroiditis are type 1 diabetes and celiac disease.[15]
Postpartum thyroiditis occurs in some females following delivery. The gland gets inflamed and the condition initially presents with over activity of the gland followed by under activity. In some cases, the gland does recover with time and resume its functions.
Enlargement of the thyroid
An enlarged thyroid gland can exist and not be considered "hyperthyroidism". The term "Non-toxic goiter" (or simply 'Goiter') is used when enlargement of the thyroid gland occurs - but only if the enlargement is not as a result of hyperthyroidism (not due to the overproduction of a thyroid hormone), nor due to a malignancy. Only then can the condition be deemed a "Non-toxic Goiter" (or 'Goiter') for short. This enlargement, the 'goiter', can occur when iodine is not in the diet in sufficient amounts. Goiter due to iodine deficiency is uncommon in developed countries as various food items come standard with added iodine (i.e., Seasoning-type table salt is supplemented with iodine). Iodine deficiency is still observed in some developing parts of the world.
In addition, enlargement of the thyroid can also occur as a result of a bacterial infection or a viral infection. When this occurs it is deemed 'Thyroiditis'.
Goiter typically takes many years to present.
Cancers
Cancers do occur in the thyroid gland and, in general, are more common in females. In most cases, the thyroid cancer presents as a painless mass in the neck. It is very unusual for the thyroid cancers to present with symptoms, unless it has been neglected. One may be able to feel a hard nodule in the neck. Diagnosis is made using a needle biopsy and various radiological studies. All thyroid cancers are treated with surgery.[16]
Non-cancerous nodules
Many individuals may find the presence of small masses (nodules) in the neck. The majority of these thyroid nodules are benign (non cancerous). The presence of a thyroid nodule does not mean one has thyroid disease. Most thyroid nodules do not cause any symptoms, and most are discovered on an incidental exam. Doctors usually perform a needle aspiration biopsy of the thyroid to determine the status of the nodules. If the nodule is found to be non-cancerous, no other treatment is required. If the nodule is suspicious then surgery is recommended..
Seasonal Aggravation
Limited research shows that seasonal allergies may trigger episodes of hypo- or hyperthyroidism.[17][18]
Physiology
The primary function of the thyroid is production of the hormones triiodothyronine (T3), thyroxine (T4), and calcitonin. Up to 80% of the T4 is converted to T3 by peripheral organs such as the liver, kidney and spleen. T3 is several times more powerful than T4, which is largely a prohormone, perhaps four[19] or even ten times more active.[20]
T3 and T4 production and action

Thyroxine (T4) is synthesised by the follicular cells from free tyrosine and on the tyrosine residues of the protein called thyroglobulin (Tg). Iodine is captured with the "iodine trap" by the hydrogen peroxide generated by the enzyme thyroid peroxidase (TPO)[22] and linked to the 3' and 5' sites of the benzene ring of the tyrosine residues on Tg, and on free tyrosine. Upon stimulation by the thyroid-stimulating hormone (TSH), the follicular cells reabsorb Tg and cleave the iodinated tyrosines from Tg in lysosomes, forming T4 and T3 (in T3, one iodine atom is absent compared to T4), and releasing them into the blood. Deiodinase enzymes convert T4 to T3.[23] Thyroid hormones that are secreted from the gland is about 80-90% T4 and about 10-20% T3.[19][20]
Cells of the brain are a major target for the thyroid hormones T3 and T4. Thyroid hormones play a particularly crucial role in brain maturation during fetal development.[24] A transport protein that seems to be important for T4 transport across the blood-brain barrier (OATP1C1) has been identified.[25] A second transport protein (MCT8) is important for T3 transport across brain cell membranes.[25]
Non-genomic actions of T4 are those that are not initiated by liganding of the hormone to intranuclear thyroid receptor. These may begin at the plasma membrane or within cytoplasm. Plasma membrane-initiated actions begin at a receptor on the integrin alphaV beta3 that activates ERK1/2. This binding culminates in local membrane actions on ion transport systems such as the Na(+)/H(+) exchanger or complex cellular events including cell proliferation. These integrins are concentrated on cells of the vasculature and on some types of tumor cells, which in part explains the proangiogenic effects of iodothyronines and proliferative actions of thyroid hormone on some cancers including gliomas. T4 also acts on the mitochondrial genome via imported isoforms of nuclear thyroid receptors to affect several mitochondrial transcription factors. Regulation of actin polymerization by T4 is critical to cell migration in neurons and glial cells and is important to brain development.
T3 can activate phosphatidylinositol 3-kinase by a mechanism that may be cytoplasmic in origin or may begin at integrin alpha V beta3.
In the blood, T4 and T3 are partially bound to thyroxine-binding globulin (TBG), transthyretin, and albumin. Only a very small fraction of the circulating hormone is free (unbound) - T4 0.03% and T3 0.3%. Only the free fraction has hormonal activity. As with the steroid hormones and retinoic acid, thyroid hormones cross the cell membrane and bind to intracellular receptors (α1, α2, β1 and β2), which act alone, in pairs or together with the retinoid X-receptor as transcription factors to modulate DNA transcription[1].
T3 and T4 regulation
The production of thyroxine and triiodothyronine is regulated by thyroid-stimulating hormone (TSH), released by the anterior pituitary. The thyroid and thyrotropes form a negative feedback loop: TSH production is suppressed when the T4 levels are high. The TSH production itself is modulated by thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus and secreted at an increased rate in situations such as cold (in which an accelerated metabolism would generate more heat). TSH production is blunted by somatostatin (SRIH), rising levels of glucocorticoids and sex hormones (estrogen and testosterone), and excessively high blood iodide concentration.
An additional hormone produced by the thyroid contributes to the regulation of blood calcium levels. Parafollicular cells produce calcitonin in response to hypercalcemia. Calcitonin stimulates movement of calcium into bone, in opposition to the effects of parathyroid hormone (PTH). However, calcitonin seems far less essential than PTH, as calcium metabolism remains clinically normal after removal of the thyroid (thyroidectomy), but not the parathyroids.
Thyroid function laboratory tests - normal ranges[26]
| Test | Abbreviation | Normal ranges |
|---|---|---|
| Serum thyrotropin/thyroid-stimulating hormone | TSH | 0.3–3.0 μU/ml |
| Free thyroxine | FT4 | 7–18 ng/l = 0.7–1.8 ng/dl |
| Serum triiodothyronine | T3 | 800 ng/l – 1.8 μg/l = 80–180 ng/dl |
| Radioactive iodine-123 uptake | RAIU | 10–30% |
| Radioiodine scan (gamma camera) | N/A | N/A - thyroid contrasted images |
| Free thyroxine fraction | FT4F | 0.03–0.005% |
| Serum thyroxine | T4 | 46–120 μg/l = 4.6–12.0 μg/dl |
| Thyroid hormone binding ratio | THBR | 0.9–1.1 |
| Free thyroxine index | FT4I | 4–11 |
| Free triiodothyronine l | FT3 | 230–619 pg/d |
| Free T3 Index | FT3I | 80–180 |
| Thyroxine-binding globulin | TBG | 12–20 ug/dl T4 +1.8 μg |
| TRH stimulation test | Peak TSH | 9–30 μIU/ml at 20–30 min. |
| Serum thyroglobulin l | Tg | 0-30 ng/m |
| Thyroid microsomal antibody titer | TMAb | Varies with method |
| Thyroglobulin antibody titer | TgAb | Varies with method |
- μU/ml = mU/l, microunit per milliliter
- ng/dl, nanograms per deciliter
- μg, micrograms
- pg/d, picograms per day
- μIU/ml = mIU/l, micro-international unit per milliliter
- See [2] for more information on medical units of measure
Significance of iodine
In areas of the world where iodine is lacking in the diet the thyroid gland can become considerably enlarged, a condition called 'endemic goitre'. Pregnant women on a diet that is severely deficient of iodine can give birth to infants who can present with thyroid hormone deficiency, manifesting in problems of physical growth and development as well as brain development (a condition referred to as 'endemic cretinism'), and is one cause of congenital hypothyroidism. In many developed countries, newborns are routinely tested for congenital hypothyroidism as part of newborn screening. Children with congenital hypothyroidism are treated supplementally with levothyroxine, which facilitates normal growth and development.
Thyroxine is critical to the regulation of metabolism and growth throughout the animal kingdom. Among amphibians, for example, administering a thyroid-blocking agent such as propylthiouracil (PTU) can prevent tadpoles from metamorphosing into frogs; in contrast, administering thyroxine will trigger metamorphosis.
Because the thyroid concentrates this element, it also concentrates various radioactive isotopes of iodine produced by nuclear fission. In the event of large accidental releases of such material into the environment, the uptake of radioactive iodine isotopes by the thyroid can, in theory, be blocked by saturating the uptake mechanism with a large surplus of non-radioactive iodine, taken in the form of potassium iodide tablets. One consequence of the Chernobyl disaster was an increase in thyroid cancers in children in the years following the accident.[27]
The use of iodised salt is an efficient way to add iodine to the diet. It has eliminated endemic cretinism in most developed countries, and some governments have made the iodination of flour, cooking oil, and salt mandatory. Potassium iodide and sodium iodide are typically used forms of supplemental iodine.
As with most substances, either too much or too little can cause problems. Recent studies on some populations are showing that excess iodine intake could cause an inceased prevelence of autoimmune thyroid disease, resulting in permanent hypothyroidism.[28]
Treatment for Hyperthyroidism
Beta blockers are used to decrease symptoms like fast heart rate, tremors, anxiety and chest palpitations, and are sometimes anti thyroid drugs used to block thyroid hormones, in particular, in the case of Graves' disease. These medications take several months to take full effect and have side-effects like skin rash or a drop in white blood cell count, which decreases the ability of the body to fight off infections. Sometimes these drugs involve frequent dosing (such as one pill each 8 hours) and require frequent doctor visits (blood tests) to track impacts and progress, and sometimes may be ineffective at free. Because of the side-effects and treatment protocol of drugs, many patients choose to undergo surgery or more commonly the use of radioactive iodine 131, a type of radioiodine. Sometimes, radioiodine is administered under a thyroid ablation procedure to severely restrict or altogether destroy the gland; the radioactive iodine is selectively taken up by the gland and gradually thins out producing cells and destroys the tissues. The treatment has been noted to be safe and effective.
Individuals that have underactivity of the thyroid gland require hormone replacement therapy. Several types of thyroid hormone replacements are available and all are very safe but need to be taken for the rest of one's life. Thyroid hormone treatment is given under the care of a physician and may take a few weeks to become effective.[29]
Surgery is sometimes used to treat overactive thyroid, thyroid nodules, and often for thyroid cancers. The surgery is quite effective but can have a few side-effects or risks:
- The nerves controlling the vocal cords can be damaged.
- The parathyroid glands that produce parathyroid hormone (PTH) can be destroyed and one can develop bleeding.
- If the entire thyroid gland is removed, one develops hypothyroidism, which entails taking hormone supplements for the rest of one's life.[30]
History
There are several findings that evidence a great interest for thyroid disorders just in the Medieval Medical School of Salerno (12th century). Rogerius Salernitanus, the Salernitan surgeon and author of "Post mundi fabricam" (around 1180) was considered at that time the surgical text par excellence all over Europe. In the chapter "De bocio" of his magnum opus, he describes several pharmacological and surgical cures, some of which nowadays are reappraised quite scientifically effective.[31]
In modern times, the thyroid was first identified by the anatomist Thomas Wharton (whose name is also eponymised in Wharton's duct of the submandibular gland) in 1656.[32]
Thyroxin was identified only in the 19th century.
In other animals
The thyroid gland is found in all vertebrates. In fish, it is, in general, located below the gills and is not always divided into distinct lobes. However, in some teleosts, patches of thyroid tissue are found elsewhere in the body, associated with the kidneys, spleen, heart, or eyes.[33]
In tetrapods, the thyroid is always found somewhere in the neck region. In most tetrapod species, there are two paired thyroid glands - that is, the right and left lobes are not joined together. However, there is only ever a single thyroid gland in most mammals, and the shape found in humans is common to many other species.[33]
In larval lampreys, the thyroid originates as an exocrine gland, secreting its hormones into the gut, and associated with the larva's filter-feeding apparatus. In the adult lamprey, the gland separates from the gut, and becomes endocrine, but this path of development may reflect the evolutionary origin of the thyroid. For instance, the closest living relatives of vertebrates, the tunicates and Amphioxus, have a structure very similar to that of larval lampreys, and this also secretes iodine-containing compounds (albeit not thyroxine).[33]
Additional images
-
Position of the Thyroid in Males and Females
-
Section of the neck at about the level of the sixth cervical vertebra.
-
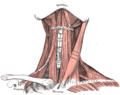
Muscles of the neck. Anterior view.
-
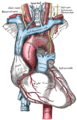
The arch of the aorta, and its branches.
-
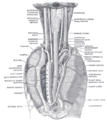
Superficial dissection of the right side of the neck, showing the carotid and subclavian arteries.
-
Diagram showing common arrangement of thyroid veins.
-
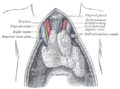
Sagittal section of nose mouth, pharynx, and larynx.
-
Muscles of the pharynx, viewed from behind, together with the associated vessels and nerves.
-
The position and relation of the esophagus in the cervical region and in the posterior mediastinum. Seen from behind.
-
Section of thyroid gland of sheep. X 160.
-
The thymus of a full-term fetus, exposed in situ.
-
Thyoid histology
See also
References
- ^ Clinical Case - Anterior Triangle of the Neck.
- ^ Yalçin B., Ozan H. (2006). "Detailed investigation of the relationship between the inferior laryngeal nerve including laryngeal branches and ligament of Berry". Journal of the American College of Surgeons. 202 (2): 291–6. doi:10.1016/j.jamcollsurg.2005.09.025. PMID 16427555.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lemaire, David (2005-05-27). "eMedicine - Thyroid anatomy". Retrieved 2008-01-19.
- ^ M. Aroon Kamath, M.D.. Are the ligaments of Berry the only reason why the thyroid moves up with deglutition?. Doctors Lounge Website. Available at: http://www.doctorslounge.com/index.php/blogs/page/13485. Accessed August 24, 2010.
- ^ Venturi, S; Donati, FM; Venturi, A; Venturi, M (2000). "Environmental iodine deficiency: A challenge to the evolution of terrestrial life?". Thyroid : official journal of the American Thyroid Association. 10 (8): 727–9. doi:10.1089/10507250050137851. PMID 11014322.
- ^ Küpper FC, Carpenter LJ, McFiggans GB; et al. (2008). "Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry" (Free full text). Proceedings of the National Academy of Sciences of the United States of America. 105 (19): 6954–8. doi:10.1073/pnas.0709959105. PMC 2383960. PMID 18458346.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Venturi, S (2010). "Thyroid Hormone, Iodine and Human Brain Evolution". Environmental Influences on Human Brain Evolution. John Wiley & Sons. pp. 105–124. ISBN 978-0-470-45268-4.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Brown-Grant, K. (1961). "Extrathyroidal iodide concentrating mechanisms" (PDF). Physiol Rev. 41: 189.
- ^ Spitzweg, C., Joba, W., Eisenmenger, W. and Heufelder, A.E. (1998). "Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acid from salivary gland, mammary gland, and gastric mucosa". J Clin Endocrinol Metab. 83: 1746. doi:10.1210/jc.83.5.1746.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Banerjee, R.K., Bose, A.K., Chakraborty, t.K., de, S.K. and datta, A.G. (1985). "Peroxidase catalysed iodotyrosine formation in dispersed cells of mouse extrathyroidal tissues". J Endocrinol. 2: 159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Page 493 (Table 33-3) in: Eugster, Erica A.; Pescovitz, Ora Hirsch (2004). Pediatric endocrinology: mechanisms, manifestations and management. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-4059-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Zoeller RT (2003). "Transplacental thyroxine and fetal brain development". J. Clin. Invest. 111 (7): 954–7. doi:10.1172/JCI18236. PMC 152596. PMID 12671044.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM. (2010). Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity. Cereb Cortex. 20(6):1462-75. PMID: 19812240.
- ^ Fawcett, Don (2002). Bloom & Fawcett's Concise Histology. New York: Arnold Publishers. pp. 257–258. ISBN 0-340-80677-X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Treatment for Thyroid disease Retrieved on 2010-02-07
- ^ Thyroid Disorders overview Merck Sharpe & Dohme. Retrieved on 2010-02-07
- ^ Yamamoto M, Shibuya N, Chen LC, Ogata E (1988). "Seasonal recurrence of transient hypothyroidism in a patient with autoimmune thyroiditis". Endocrinol. Jpn. 35 (1): 135–42. PMID 3396511.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hidaka Y, Amino N, Iwatani Y, Itoh E, Matsunaga M, Tamaki H (1993). "Recurrence of thyrotoxicosis after attack of allergic rhinitis in patients with Graves' disease". J. Clin. Endocrinol. Metab. 77 (6): 1667–70. doi:10.1210/jc.77.6.1667. PMID 8263157.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b How Your Thyroid Works: A Delicate Feedback Mechanism. Updated 2009-05-21.
- ^ a b The thyroid gland in Endocrinology: An Integrated Approach by Stephen Nussey and Saffron Whitehead (2001) Published by BIOS Scientific Publishers Ltd. ISBN 1-85996-252-1 .
- ^ References used in image are found in image article in Commons:Commons:File:Thyroid_system.png#References.
- ^ Ekholm R, Bjorkman U (1997). "Glutathione peroxidase degrades intracellular hydrogen peroxide and thereby inhibits intracellular protein iodination in thyroid epithelium". Endocrinology. 138 (7): 2871–2878. doi:10.1210/en.138.7.2871. PMID 9202230.
- ^ Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002). "Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases". Endocr Rev. 23 (1): 38–89. doi:10.1210/er.23.1.38. PMID 11844744.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kester MH, Martinez de Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, Hume R, Morreale de Escobar G (2004). "Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas". J Clin Endocrinol Metab. 89 (7): 3117–3128. doi:10.1210/jc.2003-031832. PMID 15240580.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jansen J, Friesema ECH, Milici C, Visser TJ (2005). Thyroid hormone transporters in health and disease. Thyroid 15;757-768. PMID 16131319.
- ^ http://www.endocrineweb.com/TFT.html
- ^ "Chernobyl children show DNA changes". BBC News. 2001-05-08. Retrieved 2010-05-25.
- ^ Patrick L (2008). "Iodine: deficiency and therapeutic considerations" (PDF). Altern Med Rev. 13 (2): 116–27. PMID 18590348.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thyroid Disorders Information MedicineNet. Retrieved on 2010-02-07
- ^ Thyroid Problems eMedicine Health. Retrieved on 2010-02-07
- ^ Bifulco M, Cavallo P (2007). "Thyroidology in the medieval medical school of salerno". Thyroid. 17 (1): 39–40. doi:10.1089/thy.2006.0277. PMID 17274747.
- ^ Thomas Wharton at Who Named It?
- ^ a b c Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 555–556. ISBN 0-03-910284-X.
External links
- EndocrineWeb.com for more information on thyroid disease, hormones, and surgery
- American Thyroid Association (Thyroid Information and professional organization)
- Histology at KUMC epithel-epith03 "Thyroid Gland"