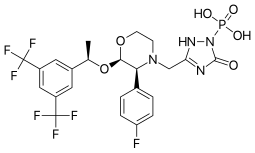
Fosaprepitant
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emend, Ivemend |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | >95% (aprepitant) |
| Metabolism | To aprepitant |
| Elimination half-life | 9 to 13 hours (aprepitant) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H22F7N4O6P |
| Molar mass | 614.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fosaprepitant (Emend for Injection (US), Ivemend (EU)) is an antiemetic medication, administered intravenously. It is a prodrug of aprepitant.
Fosaprepitant was developed by Merck & Co. and was approved by the U.S. Food and Drug Administration (FDA) on January 25, 2008,[1] and by the European Medicines Agency (EMA) on January 11 of the same year.[2]
References
- ^ "Drugs.com, FDA Approves Emend (fosaprepitant dimeglumine) for Injection, Merck's New Intravenous Therapy, for Use in Combination with Other Antiemetics for Prevention of Nausea and Vomiting Caused by Chemotherapy". Retrieved 2008-03-15.
- ^ "European Public Assessment Report for Ivemend (from the EMEA website)". Archived from the original on 2008-02-28. Retrieved 2008-03-15.
External links
- "Fosaprepitant dimeglumine". Drug Information Portal. U.S. National Library of Medicine.
