Arogenate dehydratase
| Arogenate dehydratase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 The crystal structure of Cyclohexadienyl dehydratase precursor from Pseudomonas aeruginosa PA01. The secondary structure is displayed in the image, and the residue important for catalysis has been highlighted. | |||||||||
| Identifiers | |||||||||
| EC no. | 4.2.1.91 | ||||||||
| CAS no. | 76600-70-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
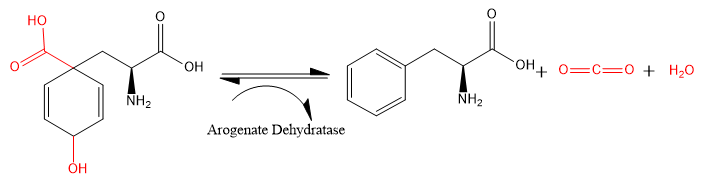
Arogenate dehydratase (ADT) (EC 4.2.1.91) is an enzyme that catalyzes the chemical reaction
- L-arogenate → L phenylalanine + H2O + CO2
Certain forms of the protein have the potential to catalyze a second reaction,[1]
- L-prephenate → L-phenylpyruvate + H2O + CO2
This enzyme participates in phenylalanine, tyrosine, and tryptophan biosynthesis (an example structure is shown to the right.[2]
Nomenclature
This enzyme belongs to the family of lyases, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is L-arogenate hydro-lyase (decarboxylating; L-phenylalanine-forming). Other names in common use include:
- arogenate dehydratase
- L-arogenate hydro-lyase (decarboxylating)
- cyclohexadienyl dehydratase
- carbocyclohexadienyl dehydratase
- pheC
- ADT
Reaction
The carboxyl and hydroxide groups (shown in red) attached to the 2,5-cyclohexene ring are eliminated from L-arogenate, leaving as carbon dioxide and water. The 2,5-cyclohexene ring becomes a phenyl ring, and L-phenylalanine is formed.
Certain forms of ADT have been shown to exhibit some prephenate dehydratase (PDT) activity in addition to the standard ADT activity described above.[1] Known as cyclohexadienyl dehydratases or carbocyclohexadienyl dehydratases (listed above),[1] these forms of the enzyme catalyze the same type of reaction (a decarboxylation and a dehydration) on prephenate. The carboxyl and hydroxide groups (in red) attached to the 2,5-cyclohexene ring are removed, leaving phenylpyruvate.

Function
ADT catalyzes a reaction categorized by two major changes in the structure of the substrate, these being a decarboxylation and a dehydration; the enzyme removes a carboxyl group and a water molecule (respectively).[1] Both potential products of this reaction (L-arogenate and phenylpyruvate) occur at or near the end of the biosynthetic pathway. Total synthesis of L-arogenate has been reported.[3][4]
Structure
The structure of arogenate dehydratases are described as having, for the most part, three major sections. ADTs contain an N-terminal transit peptide, a PDT-like domain, and an ACT (Aspartokinase, chorismate mutase, TyrA) domain.[5]
Homologues
Homologues for ADT have been isolated in Arabidopsis thaliana (rabbit-ear cress),[5] Nicotiana sylvestris (tobacco),[6] Spinacia oleracea (spinach),[6] Petunia hybrida,[7] Sorghum bicolor,[8] Oryza sativa,[9] and Pinus pinaster[10] which are all considered higher-order plants. Erwinia herbicola[11] and Pseudomonas aeruginosa[12] are known to have homologues for cyclohexadienyl dehydratase. Of the plants with ADT homologues, both Arabidopsis thaliana, Petunia hybrida, and Pinus pinaster are known to have paralogues for the gene (six, three, and nine, respectively).[5][7][10]
References
- ^ a b c d Fischer R, Jensen R (1987). "[59] Arogenate dehydratase". Metabolism of Aromatic Amino Acids and Amines. Methods in Enzymology. Vol. 142. pp. 495–502. doi:10.1016/S0076-6879(87)42061-2. ISBN 9780121820428. PMID 3600377.
{{cite book}}:|journal=ignored (help) - ^ Tan K, Marshall N, Buck K, Joachimiak A (2009). "The crystal structure of cyclohexadienyl dehydratase precursor from Pseudomonas aeruginosa PA01". doi:10.2210/pdb3kbr/pdb.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Crossley MJ, Reid RC (1994). "Concise synthesis of arogenate. A biosynthetic precursor of phenylalanine and tyrosine". Journal of the Chemical Society, Chemical Communications (19): 2237–2238. doi:10.1039/c39940002237.
- ^ Danishefsky S, Morris J, Clizbe LA (2002-05-01). "Total synthesis of pretyrosine (arogenate)". Journal of the American Chemical Society. 103 (6): 1602–1604. doi:10.1021/ja00396a070.
- ^ a b c Cho MH, Corea OR, Yang H, Bedgar DL, Laskar DD, Anterola AM, et al. (October 2007). "Phenylalanine biosynthesis in Arabidopsis thaliana. Identification and characterization of arogenate dehydratases". The Journal of Biological Chemistry. 282 (42): 30827–35. doi:10.1074/jbc.m702662200. PMID 17726025.
- ^ a b Jung E, Zamir LO, Jensen RA (October 1986). "Chloroplasts of higher plants synthesize L-phenylalanine via L-arogenate". Proceedings of the National Academy of Sciences of the United States of America. 83 (19): 7231–5. Bibcode:1986PNAS...83.7231J. doi:10.1073/pnas.83.19.7231. PMC 386689. PMID 3463961.
- ^ a b Maeda H, Shasany AK, Schnepp J, Orlova I, Taguchi G, Cooper BR, et al. (March 2010). "RNAi suppression of Arogenate Dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals". The Plant Cell. 22 (3): 832–49. doi:10.1105/tpc.109.073247. PMC 2861463. PMID 20215586.
- ^ Siehl DL, Conn EE (February 1988). "Kinetic and regulatory properties of arogenate dehydratase in seedlings of Sorghum bicolor (L.) Moench". Archives of Biochemistry and Biophysics. 260 (2): 822–9. doi:10.1016/0003-9861(88)90513-9. PMID 3124763.
- ^ Yamada T, Matsuda F, Kasai K, Fukuoka S, Kitamura K, Tozawa Y, et al. (May 2008). "Mutation of a rice gene encoding a phenylalanine biosynthetic enzyme results in accumulation of phenylalanine and tryptophan". The Plant Cell. 20 (5): 1316–29. doi:10.1105/tpc.107.057455. PMC 2438470. PMID 18487352.
- ^ a b El-Azaz J, de la Torre F, Ávila C, Cánovas FM (July 2016). "Identification of a small protein domain present in all plant lineages that confers high prephenate dehydratase activity". The Plant Journal. 87 (2): 215–29. doi:10.1111/tpj.13195. PMID 27125254.
- ^ Xia TH, Ahmad S, Zhao GS, Jensen RA (May 1991). "A single cyclohexadienyl dehydratase specifies the prephenate dehydratase and arogenate dehydratase components of one of two independent pathways to L-phenylalanine in Erwinia herbicola". Archives of Biochemistry and Biophysics. 286 (2): 461–5. doi:10.1016/0003-9861(91)90066-r. PMID 1897969.
- ^ Zhao GS, Xia TH, Fischer RS, Jensen RA (February 1992). "Cyclohexadienyl dehydratase from Pseudomonas aeruginosa. Molecular cloning of the gene and characterization of the gene product". The Journal of Biological Chemistry. 267 (4): 2487–93. doi:10.1016/S0021-9258(18)45905-4. PMID 1733946.
