Combretol
Appearance
| |
| Names | |
|---|---|
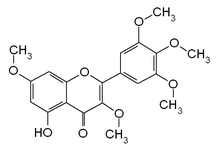
| IUPAC name
5-hydroxy-3,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one
| |
| Other names
5-Hydroxy-3,3',4',5',7-pentamethoxyflavone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H20O8 | |
| Molar mass | 388.36 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Combretol is an O-methylated flavonol, a type of flavonoid. It is the 3,7,3',4',5'-O-methylation of myricetin and can be extracted from Combretum quadrangulare[1] and from Rhodomyrtus tomentosa.[2]
References
- ^ Combretol from Combretum quadrangulare. Stang Mongkolsuk, F. M. Dean and L. E. Houghton, J. Chem. Soc. C, 1966, page 125, doi:10.1039/J39660000125
- ^ 5-Hydroxy-3,3',4',5',7-pentamethoxyflavone (combretol). Dachriyanus, R. Fahmi, M. V. Sargent, B. W. Skelton and A. H. White, Acta Crystallogr. (2004). E60, o86-o88
