Disodium tetracarbonylferrate
| |
| Names | |
|---|---|
| IUPAC name
disodium tetracarbonylferrate
| |
| Systematic IUPAC name
disodium tetracarbonylferrate | |
| Other names
disodium iron tetracarbonyl,
Collman's reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.035.395 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4FeNa2O4 | |
| Molar mass | 213.87 |
| Appearance | Colorless solid |
| Density | 2.16 g/cm3, solid |
| Decomposes | |
| Solubility | tetrahydrofuran, dimethylformamide, dioxane |
| Structure | |
| Distorted tetrahedron | |
| Tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Pyrophoric |
| Related compounds | |
Related compounds
|
Iron pentacarbonyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
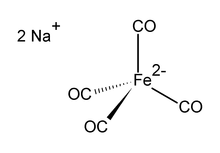
Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g. with tetrahydrofuran or dimethoxyethane which bind to the sodium cation.[1] It is mainly used to synthesise aldehydes.[2] This oxygen-sensitive colourless solid is a reagent in organometallic and organic chemical research but is not of commercial value.
Structure
The dianion [Fe(CO)4]2− is isoelectronic with Ni(CO)4 and does adopt a tetrahedral structure in its salts.
It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's reagent, in recognition of James P. Collman an early popularizer of its use.[3] The tetracarbonylferrate dianion is tetrahedral.[4][5]
Synthesis
The reagent was first prepared by reducing iron pentacarbonyl with sodium amalgam.[6] A more modern synthesis uses sodium naphthenide as the reducant. The efficiency of the synthesis depends on the quality of the iron pentacarbonyl.[1]
- Fe(CO)5 + 2 Na → Na2[Fe(CO)4] + CO
When a deficiency of sodium is used, the reduction affords octacarbonyl diferrate:[1]
- 2 Fe(CO)5 + 2 Na → Na2[Fe2(CO)8] + 2 CO
Other reducing agents work. Another way to synthesize Collman's Reagent is to use FeCl3.[7]
- FeCl3 + Na(C10H8) + 4CO + THF → +Na → Na2[Fe(CO)4]
These synthesis pathways are extremely useful in preparing Collman's Reagent if the typical reagents to make it are not available.[8]
Reactions
The reagent was originally described for the conversion of primary alkyl bromides to the corresponding aldehydes in a two-step, "one-pot" reaction:[6]
- Na2[Fe(CO)4] + RBr → Na[RFe(CO)4] + NaBr
This solution is then treated sequentially with PPh3 and then acetic acid to give the aldehyde, RCHO.
Disodium tetracarbonylferrate can be used to convert acid chlorides to aldehydes. This reaction proceeds via the intermediacy of iron acyl complex.
- Na2[Fe(CO)4] + RCOCl → Na[RC(O)Fe(CO)4] + NaCl
- Na[RC(O)Fe(CO)4] + HCl → RCHO + "Fe(CO)4" + NaCl
Disodium tetracarbonylferrate reacts with alkyl halides (RX) to produce alkyl complexes:
- Na2[Fe(CO)4] + RX → Na[RFe(CO)4] + NaX
Such iron alkyls can be converted to the corresponding carboxylic acid and acid halides:
- Na[RFe(CO)4] + O2, H+ →→ RCO2H + Fe...
- Na[RFe(CO)4] + 2 X2 → RC(O)X + FeX2 + 3 CO + NaX
References
- ^ a b c Strong, H.; Krusic, P. J.; San Filippo, J. (1990). R. J. Angelici (ed.). "Sodium Carbonyl Ferrates, Na2[Fe(CO)4], Na2[Fe2(CO)8], and Na2[Fe3(CO)11]. Bis[μ-Nitrido-Bis(triphenylphosphorus)1+] Undeca-Carbonyltriferrate2−, [(Ph3P)2N]2[Fe3(CO)11]". Inorganic Syntheses. 28. New York: J. Wiley & Sons: 203–207. doi:10.1002/9780470132593.ch52. ISBN 0-471-52619-3.
- ^ Pike, R. D. (2001). "Disodium Tetracarbonylferrate(-II)". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd465.
- ^ Miessler, G. L.; Tarr, D. A. (2004). Inorganic Chemistry. Upper Saddle River, NJ: Pearson.
- ^ Chin, H. B.; Bau, R. (1976). "The Crystal Structure of Disodium Tetracarbonylferrate. Distortion of the Tetracarbonylferrate2− Anion in the Solid State". Journal of the American Chemical Society. 98 (9): 2434–2439. doi:10.1021/ja00425a009.
- ^ "Dependence of the tetracarbonylferrate(2-) geometry on counterion: crystal structures of dipotassium tetracarbonylferrate and bis(sodium crypt) tetracarbonylferrate [crypt = N(CH2CH2OCH2CH2OCH2CH2)3N]". Journal of the American Chemical Society. 99: 1104–1111. 1977. doi:10.1021/ja00446a022.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Cooke, M. P. (1970). "Facile Conversion of Alkyl Bromides into Aldehydes Using Sodium Tetracarbonylferrate(-II)". Journal of the American Chemical Society. 92 (20): 6080–6082. doi:10.1021/ja00723a056.
- ^ Scholsser, M. (2013). Organometallics in Synthesis, Third Manual. Chicester, England: Wiley.
- ^ Rameshkumar, C. (2011). New Reactive Iron Carbonyl Reagents for Applications in Organic Synthesis. Hyderabad, India: University of Hyderabad.
Further reading
- Collman, J. P. (1975). "Disodium Tetracarbonylferrate, a Transition Metal Analog of a Grignard Reagent". Accounts of Chemical Research. 8 (10): 342–347. doi:10.1021/ar50094a004.
- Ungurenasu, C.; Cotzur, C. (1982). "Disodium Tetracarbonylferrate: A Reagent for Acid Functionalization of Halogenated Polymers". Polymer Bulletin. 6 (5–6): 299–303. doi:10.1007/BF00255401.
- Hieber, V. W.; Braun, G. (1959). "Notizen: "Rheniumcarbonylwasserstoff" und Methylpentacarbonylrhenium". Zeitschrift für Naturforschung B. 14 (2): 132–133. doi:10.1515/znb-1959-0214.
