Phytoene desaturase (lycopene-forming)
| Phytoene desaturase (lycopene-forming) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystallographic structure of a bacterial phytoene desaturase monomer from Pantoea ananatis.[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 1.3.99.31 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Phytoene desaturase (lycopene-forming) (CrtI, four-step phytoene desaturase) (EC 1.3.99.31, 15-cis-phytoene:acceptor oxidoreductase (lycopene-forming)) are enzymes found in archaea, bacteria and fungi that are involved in carotenoid biosynthesis.[2] They catalyze the conversion of colorless 15-cis-phytoene into a bright red lycopene in a biochemical pathway called the poly-trans pathway. The same process in plants and cyanobacteria utilizes four separate enzymes in a poly-cis pathway.[3]
Biochemistry
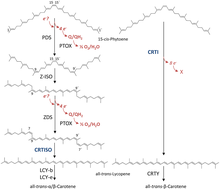
Bacterial phytoene desaturases were shown to require FAD as a cofactor for their function.[4] During the chemical reaction in total four additional double bonds are introduced into phytoene:
- 15-cis-phytoene + 4 acceptor all-trans-lycopene + 4 reduced acceptor (overall reaction)
- (1a) 15-cis-phytoene + acceptor all-trans-phytofluene + reduced acceptor
- (1b) all-trans-phytofluene + acceptor all-trans-zeta-carotene + reduced acceptor
- (1c) all-trans-zeta-carotene + acceptor all-trans-neurosporene + reduced acceptor:
- (1d) all-trans-neurosporene + acceptor all-trans-lycopene + reduced acceptor
Applications
In 2000 it was discovered that the gene insertion of a bacterial phytoene desaturase into transgenic tomatoes increased the lycopene content without the need to alter several of the plants enzymes.[5] This approach was later used in rice to increase its β-carotene content resulting in the Golden Rice project.
See also
- 15-''Cis''-phytoene desaturase
- Phytoene desaturase (neurosporene-forming)
- Phytoene desaturase (zeta-carotene-forming)
- Phytoene desaturase (3,4-didehydrolycopene-forming)
References
- ^ PDB: 4dgk; Schaub P; Yu Q; Gemmecker S; Poussin-Courmontagne P; Mailliot J; McEwen AG; Ghisla S; Al-Babili S; Cavarelli J; Beyer P (2012). "On the Structure and Function of the Phytoene Desaturase CRTI from Pantoea ananatis, a Membrane-Peripheral and FAD-Dependent Oxidase/Isomerase". PLOS ONE. 7 (6): e39550. doi:10.1371/journal.pone.0039550. PMC 3382138. PMID 22745782.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fraser, P.D.; Misawa, N.; Linden, H.; Yamano, S.; Kobayashi, K.; Sandmann, G. (1992). "Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase". J. Biol. Chem. 267 (28): 19891–19895. PMID 1400305.
- ^ Moise AR, Al-Babili S, Wurtzel ET (31 October 2013). "Mechanistic aspects of carotenoid biosynthesis". Chemical Reviews. 114 (1): 164–193. doi:10.1021/cr400106y. PMC 3898671. PMID 24175570.
- ^ Dailey TA; Dailey HA (May 1998). "Identification of an FAD superfamily containing protoporphyrinogen oxidases, monoamine oxidases, and phytoene desaturase. Expression and characterization of phytoene desaturase of Myxococcus xanthus". Journal of Biological Chemistry. 273 (22): 13658–13662. doi:10.1074/jbc.273.22.13658. PMID 9593705.
- ^ Romer, S.; Fraser, P.D.; Kiano, J.W.; Shipton, C.A.; Misawa, N; Schuch, W.; Bramley, P.M. (2000). "Elevation of provitamin A content of transgenic tomato plants". Nature Biotechnology. 18 (6): 666–669. doi:10.1038/76523. PMID 10835607.
External links
- Phytoene+desaturase+(lycopene-forming) at the U.S. National Library of Medicine Medical Subject Headings (MeSH)

