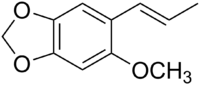
Carpacin
Appearance
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Methoxy-6-[(1E)-prop-1-en-1-yl]-2H-1,3-benzodioxole | |
| Other names
(E)-5-Methoxy-6-(prop-1-en-1-yl)benzo[d][1,3]dioxole
(E)-2-Methoxy-4,5-methylenedioxypropenylbenzene (no longer recommended) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12O3 | |
| Molar mass | 192.21 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Carpacin is a naturally occurring organic compound first isolated from the Carpano tree (an unidentified Cinnamomum species of the family Lauraceae which is native to Bougainville Island), from which it derives its name. It is a biosynthetic precursor of the more complex lignan-dimer, carpanone.[1] Carpacin is classified as a phenylpropanoid.
Carpacin has been prepared synthetically from sesamol[2] and has been studied for potential use as an insecticide,[3] an antidepressant, and an inhibitor of carcinogenesis.[2]
References
- ^ J. Mohandas; M. Slaytor; T.R. Watson (1969). "Trans-2-methoxy-4,5-methylenedioxypropenylbenzene (carpacin) from a Cinnamomum sp. from Bougainville". Aust. J. Chem. 22 (8): 1803–1804. doi:10.1071/CH9691803.
- ^ a b Tsui-Hwa Tseng; Yen-Min Tsheng; Yean-Jang Lee; Hsing-Ling Hsu (2000). "Total Synthesis of Carpacin and Its Geometric Isomer as a Cancer Chemopreventer" (PDF). Journal of the Chinese Chemical Society. 47: 1165–1169. Archived from the original (PDF) on 2005-09-14.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ BH Alexander; SI Gertler; RT Brown; TA Oda; M Beroza (1959). "Synthesis of Methylenedioxyphenyl Compounds from Isosafrole and Sesamol". J. Org. Chem. 24 (10): 1504. doi:10.1021/jo01092a029.
