Ephedra (plant)
| Ephedra | |
|---|---|

| |
| Ephedra fragilis in Mallorca | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | Ephedrales |
| Family: | Ephedraceae |
| Genus: | Ephedra |
| |
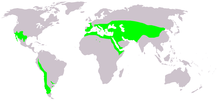
| Global range of Ephedra | |
| Synonyms[3] | |
|
Chaetocladus J.Nelson | |
Ephedra is a genus of gymnosperm shrubs, the only genus in its family, Ephedraceae, and order, Ephedrales. The various species of Ephedra are widespread in many lands, native to southwestern North America, southern Europe, northern Africa, and southwest and central Asia, northern China, and western South America.[3]
In temperate climates, most Ephedra species grow on shores or in sandy soils with direct sun exposure. Common names in English include joint-pine, jointfir, Mormon-tea or Brigham tea. The Chinese name for Ephedra species is mahuang (simplified Chinese: 麻黄; traditional Chinese: 麻黃; pinyin: máhuáng; Wade–Giles: ma-huang; lit. 'cannabis yellow'). Ephedra is also sometimes called sea grape (from the French raisin de mer), a common name for the flowering plant Coccoloba uvifera.



Medical uses

Plants of the genus Ephedra, including E. sinica and others, have traditionally been used by indigenous people for a variety of medicinal purposes, including treatment of asthma, hay fever, and the common cold.[4] The alkaloids ephedrine and pseudoephedrine are active constituents of E. sinica and other members of the genus. These compounds are sympathomimetics with stimulant and decongestant qualities and are chemically substituted amphetamines.
Pollen of Ephedra spp. was found in the Shanidar IV burial site in Iraq, which led to the suggestion that its use as a medicinal plant dates to over 60,000 years ago.[5] Paul B. Pettitt has stated that "[a] recent examination of the microfauna from the strata into which the grave was cut suggests that the pollen was deposited by the burrowing rodent Meriones persicus, which is common in the Shanidar microfauna and whose burrowing activity can be observed today".[6] It has been suggested that Ephedra may be the Soma plant of Indo-Iranian religion.[7]
Adverse Effects
Alkaloids obtained from the species of Ephedra in herbal medicines containing synthetically prepared pseudoephedrine and ephedrine can cause cardiovascular events. These events have been associated with arrhythmias, palpitations, tachycardia and myocardial infarction. Caffeine consumption in combination with ephedrine has been reported to increase the risk of these cardiovascular events.[8]
Species
Accepted species:[3]
- Ephedra alata Decne – North Africa, Arabian Peninsula
- Ephedra altissima Desf. – North Africa, Canary Islands
- Ephedra americana Humb. & Bonpl. ex Willd. – Bolivia, Ecuador, Peru, Argentina, Chile
- Ephedra antisyphilitica Berland ex C.A.Meyer – Clapweed, Erect Ephedra – Texas, Oklahoma, New Mexico, Nuevo León, Coahuila, Chihuahua
- Ephedra aphylla Forssk. – eastern Mediterranean from Libya and Cyprus to the Persian Gulf
- Ephedra × arenicola H.C.Cutler – Arizona, Utah (hybrid, E. cutleri × E. torreyana)
- Ephedra aspera Engelm. ex S.Wats. – Boundary Ephedra, Pitamoreal – Texas, New Mexico, Arizona, Utah, Nevada, California, Chihuahua, Durango, Zacatecas, Sinaloa, Sonora, Baja California
- Ephedra aurantiaca Takht. & Pachom. – Caucasus, Kazakhstan, Turkmenistan
- Ephedra boelckei F.A.Roig – Argentina
- Ephedra botschantzevii Pachom. – Kazakhstan, Tuva region of Siberia
- Ephedra breana Phil. – Peru, Bolivia, Chile, Argentina
- Ephedra brevifoliata Ghahr. – Iran
- Ephedra californica S.Wats. – California Ephedra, California Jointfir – California, western Arizona, Baja California
- Ephedra chilensis C.Presl – Chile, Argentina
- Ephedra compacta Rose – widespread in much of Mexico
- Ephedra coryi E.L.Reed – Cory's Ephedra – Texas, New Mexico
- Ephedra cutleri Peebles – Navajo Ephedra, Cutler's Ephedra, Cutler Mormon-tea, Cutler's Jointfir – Colorado, Utah, Arizona, New Mexico, Wyoming
- Ephedra dahurica Turcz. – Siberia, Mongolia
- Ephedra dawuensis Y.Yang – Sichuan
- Ephedra distachya L. – Joint-pine, Jointfir – southern Europe and central Asia from Portugal to Kazakhstan
- Ephedra × eleutherolepis V.A.Nikitin – Tajikistan (hybrid E. intermedia × E. strobilacea)
- Ephedra equisetina Bunge – Ma huang – Caucasus, Central Asia, Siberia, Mongolia, Gansu, Hebei, Inner Mongolia, Ningxia, Qinghai, Shanxi, Xinjiang
- Ephedra fasciculata A.Nelson – Arizona Ephedra, Arizona Jointfir, Desert Mormon-tea – Arizona, California, Nevada, Utah
- Ephedra fedtschenkoae Paulsen – Central Asia, Siberia, Mongolia, Xinjiang
- Ephedra foeminea Forssk. – North Africa, Somalia, Balkans, Italy, Middle East; naturalized in Santa Barbara County of California
- Ephedra foliata Boiss. ex C.A.Mey. – North Africa, Somalia, Middle East, India
- Ephedra fragilis Desf. – Mediterranean, Canary Islands, Madeira
- Ephedra frustillata Miers – Patagonian Ephedra – Chile, Argentina
- Ephedra funerea Coville & Morton – Death Valley Ephedra, Death Valley Jointfir – California, Arizona, Nevada
- Ephedra gerardiana Wallich ex C.A.Meyer – Gerard's Jointfir, Shan Ling Ma Huang – Himalayas, Tibet, Yunnan, Siberia, Central Asia
- Ephedra glauca Regel – Iran, Central Asia, Mongolia
- Ephedra holoptera Riedl – Iran
- Ephedra intermedia Schrenk & C.A.Meyer – China, Siberia, Central Asia, Himalayas, Iran, Pakistan
- Ephedra × intermixta H.C.Cutler – New Mexico (hybrid E. trifurca × E. torreyana)
- Ephedra kardangensis P.Sharma & P.L.Uniyal – western Himalayas
- Ephedra khurikensis P.Sharma & P.L.Uniyal – western Himalayas
- Ephedra laristanica Assadi – Iran
- Ephedra likiangensis Florin – Guizhou, Sichuan, Tibet, Yunnan
- Ephedra lomatolepis Schrenk – Kazakhstan, Tuva region of Siberia
- Ephedra major Host – Mediterranean, Middle East, Central Asia; from Canary Islands to Kashmir
- Ephedra milleri Freitag & Maier-St. – Oman, Yemen
- Ephedra minuta Florin – Qinghai, Sichuan
- Ephedra monosperma C.A.Meyer – Siberia, Mongolia, much of China including Tibet and Xinjiang
- Ephedra multiflora Phil. ex Stapf – Chile, Argentina
- Ephedra nevadensis S.Wats. – Nevada Ephedra, Nevada Jointfir, Nevada Mormon-tea – Baja California, California, Arizona, Nevada, Utah, Oregon
- Ephedra ochreata Miers – Argentina
- Ephedra oxyphylla Riedl – Afghanistan
- Ephedra pachyclada Boiss. – Middle East from Sinai and Yemen to Pakistan
- Ephedra pedunculata Engelm. ex S.Wats. – Vine Ephedra, Vine Jointfir – Texas, Chihuahua, Coahuila, Durango, San Luis Potosí, Nuevo León, Zacatecas
- Ephedra pentandra Pachom. – Iran
- Ephedra przewalskii Stapf – Central Asia, Mongolia, Pakistan, Gansu, Inner Mongolia, Ningxia, Qinghai, Tibet
- Ephedra pseudodistachya Pachom. – Siberia, Mongolia
- Ephedra regeliana Florin – Xi Zi Ma Huang – Central Asia, Siberia, Pakistan, Xinjiang
- Ephedra rhytidosperma Pachom. – Gansu, Inner Mongolia, Ningxia, Mongolia
- Ephedra rituensis Y.Yang, D.Z.Fu & G.H.Zhu – Qinghai, Xinjiang, Tibet
- Ephedra rupestris Benth. – Ecuador, Peru, Bolivia, Argentina
- Ephedra sarcocarpa Aitch. & Hemsl. -Pakiostan, Afghanistan
- Ephedra sinica Stapf – Cao Ma Huang, Chinese ephedra – Mongolia, Siberia, Primorye, Manchuria
- Ephedra somalensis Freitag & Maier-St. – Somalia, Eritrea
- Ephedra strobilacea Bunge – Iran, Central Asia
- Ephedra sumlingensis P.Sharma & P.L.Uniyal – western Himalayas
- Ephedra tilhoana Maire – Chad
- Ephedra torreyana S.Wats. – Torrey's Ephedra, Torrey's Jointfir, Torrey's Mormon-tea, Cañutillo – Nevada, Utah, Colorado, Arizona, New Mexico, Texas, Chihuahua
- Ephedra transitoria Riedl – Iraq, Syria, Palestine, Saudi Arabia
- Ephedra triandra Tul. -Bolivia, Argentina
- Ephedra trifurca Torrey ex S.Wats. – Longleaf Ephedra, Longleaf Jointfir, Longleaf Mormon-tea, Popotilla, Teposote – California, Arizona, New Mexico, Texas, Chihuahua, Sonora, Baja California
- Ephedra tweedieana C.A.Mey. – Brazil, Argentina, Uruguay
- Ephedra viridis Coville – Green Ephedra, Green Mormon-tea – California, Nevada, Utah, Arizona, New Mexico, Colorado, Wyoming, South Dakota, Oregon
- Ephedra vvedenskyi Pachom. – Iran, Caucasus, Turkmenistan
Economic botany and alkaloid content
Earliest uses of Ephedra spp. (mahuang) for specific illnesses date back to 5000 BC. Ephedrine and isomers were already isolated in 1881 from Ephedra dystachia and characterized by the Japanese organic chemist Nagai Nagayoshi of the 19th century. His work to access Ephedra drug materials to isolate a pure pharmaceutical substance, and the systematic production of semi-synthetic derivatives thereof is relevant still today as the three species Ephedra sinica, Ephedra vulgaris and to a lesser extent Ephedra equisetina are commercially grown in Mainland China as a source for natural ephedrines and isomers for use in pharmacy. E. sinica and E. vulgaris usually carry six optically active phenylethylamines, mostly ephedrine and pseudoephedrine with minor amounts of norephedrine, norpseudoephedrine as well as the three methylated analogs. Reliable information on the total alkaloid content of the crude drug is difficult to obtain. Based on HPLC analyses in industrial settings, the concentrations of total alkaloids in dried Herba Ephedra ranged between 1 and 4%, and in some cases up to 6%.[9]
For a review of the alkaloid distribution in different species of the genus Ephedra see Jian-fang Cui (1991).[10] Other American and European species of Ephedra, e.g. Ephedra nevadensis (Nevada Mormon tea) have not been systematically assayed; based on unpublished field investigations, they contain very low levels (less than 0.1%) or none at all.[11]
References
- ^ "Ephedrales Dumort". EU-NOMEN. Retrieved 20 January 2016.
- ^ a b Kramer, K.U.; Green, P.S., eds. (1990). The Families and Genera of Vascular Plants, Vol. 1: Pteridophytes and Gymnosperms. Berlin: Springer-Verlag. pp. 379–381. ISBN 3540517944.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ a b c Kew World Checklist of Selected Plant Families
- ^ Abourashed E, El-Alfy A, Khan I, Walker L (2003). "Ephedra in perspective—a current review". Phytother Res. 17 (7): 703–12. doi:10.1002/ptr.1337. PMID 12916063.
- ^ Solecki, Ralph S. (1975). "Shanidar IV, a Neanderthal Flower Burial in Northern Iraq". Science. 190 (4217): 880–881. doi:10.1126/science.190.4217.880. JSTOR 1741776.
- ^ Paul B. Pettitt (2002). "The Neanderthal dead: exploring mortuary variability in Middle Palaeolithic Eurasia". Before Farming. 1 (4): 1–26.
- ^ Rudgley, Richard (1993). The Alchemy of Culture. London: British Museum Press. pp. 44–45. ISBN 0-7141-2711-6.
- ^ Skalli, Souad; Zaid, Abdelhamid; Soulaymani, Rachida (December 2007). "Drug Interactions With Herbal Medicines". Ther Drug Monit. 29 (6): 1–8.
- ^ Brossi, Arnold (ed) (1989), The Alkaloids: Chemistry and Pharmacology, Vol. 35, ISBN 0-12-469535-3.
- ^ Cui, Jian-fang; et al. (1991). "Analysis of alkaloids in Chinese Ephedra species by GC methods". Phytochemical Analysis. 2 (3): 116–119. doi:10.1002/pca.2800020305.
- ^ Hegnauer R. (1962) "Chemotaxonomie der Pflanzen. I". Birkhauser Verlag, Basel; Switzerland, pp. 460–462 as cited in Roman MC (2004). "Determination of ephedrine alkaloids in botanicals and dietary supplements by HPLC-UV: collaborative study". J AOAC Int. 87 (1): 1–14. PMID 15084081.
External links
- Ephedra viridis (Plants for a Future Database)
- Usage in Chinese Medicine
- Ephedra fact sheet, NIH National Center for Complementary and Integrative Health
- Ephedrea (Evidence and dosing), Mayo Clinic
- Ephedra - Clinical summary and mechanism of action, MSKCC Memorial Sloan Kettering Cancer Center
- Ephedraceae of Mongolia in FloraGREIF
