Glycerol-1,2-carbonate

| |
| Names | |
|---|---|
| Other names
* 4-Hydroxymethyl-1,3-dioxolan-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.032 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O4 | |
| Molar mass | 118,09 g·mol−1 |
| Appearance | clear, colourless[2] to light yellow[3] liquid |
| Density | * 1,40 g·cm−3 at 25 °C[2]
|
| Melting point | * −60 °C at 1013 hPa[4]
|
| Boiling point | * 110–115 °C at 0.1 mmHg[5] |
| with water[5] and polar solvents such as alcohols and miscible with Tetrahydrofuran[6] | |
| Vapor pressure | 0.93 Pa at 25 °C[4] |
Refractive index (nD)
|
* 1,4570 – 1,4610 (20 °C)[3]
|
| Hazards[7] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glycerol-1,2-carbonate is formally the cyclic ester of carbonic acid with glycerol and has aroused great interest as a possible product from the "waste materials" carbon dioxide CO2 and glycerol (especially from biodiesel production) with a wide range of applications.[9]
The currently unsatisfactory yields and complex process conditions[10] in direct synthesis stand in the way of a further spread of glycerol carbonate as a solvent and molecular building block from renewable raw materials.
Manufacture
[edit]A large number of synthetic routes have been described for the production of glycerol-1,2-carbonate,[11][12] many of which do not meet the criteria for modern chemical processes in terms of economy, environmental compatibility and safety.
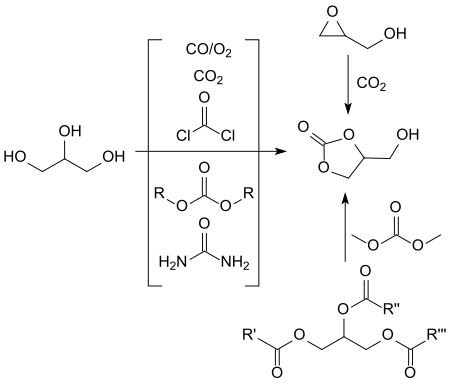
Non-glycerol-based syntheses, especially if they are based on expensive precursors such as glycidol[13] or 3-chloro-1,2-propanediol or epichlorohydrin[12] and expensive catalysts make no contribution to the economic solution of the huge glycerol oversupply (estimate for 2024: approx. 6.3 million tons global production volume).[14]
Of the indirect glycerol-based syntheses, in which the cyclic carbonic acid ester glycerol carbonate is produced by inserting a carbonyl group, reactions with particularly inexpensive urea and with dialkyl carbonates such as dimethyl carbonate DMC and ethylene carbonate have been particularly studied.

The transesterification produces methanol or, in the case of urea, ammonia as a by-product, which can be easily removed from the reaction and have little disruption to the work-up.
Recently, a glycerol carbonate synthesis with urea under microwave irradiation with anhydrous zinc sulfate as a heterogeneous catalyst was described. Under optimized conditions (150 °C, 100 min reaction time), glycerol carbonate could be obtained in 93.7% yield.[15]
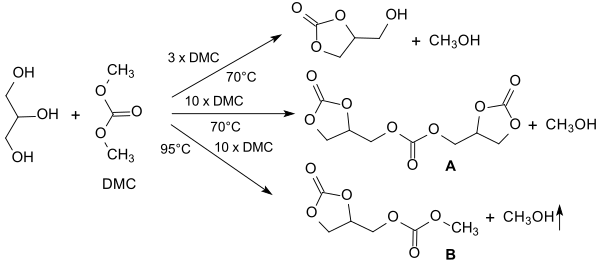
Most frequently, transesterifications of dialkyl carbonates, predominantly of dimethyl carbonate, with acidic[16] and especially basic[17] catalysts under homogeneous[18][19] and heterogeneous[9] catalysis have been investigated. Practically quantitative yields of glycerol 1,2-carbonate are sometimes achieved in this way. In homogeneous catalysis, however, it is often difficult to separate mixtures, while the solid bases used in heterogeneous catalysis are often quickly exhausted and only costly, e.g. B. by calcination at temperatures of 300 °C,[10] are recyclable. In addition, with high DMC excesses and long reaction times, by-products such as the so-called diglyceryl tricarbonate (A) and glycerol dicarbonate (B) are formed as a result of the reaction of DMC with the free hydroxyl group of the glycerol carbonate.[18]
The enzymatically catalyzed conversion of glycerol with DMC in polar solvents has also been described.[6]
Recently, continuous processes have also been published.[20] Quite high space-time yields could be achieved at moderate temperatures (140 °C) and short residence times (<10 min). The efficient direct synthesis of glycerol-1,2-carbonate from glycerol and inert CO2 remains a major challenge.

Reports of yields of up to 35% in the reaction in methanol with dibutyltin oxide as a catalyst at 80 °C[21] could not be confirmed.[22]
The results actually achieved are a maximum of 17% when using acetonitrile, which reacts in the basic (potassium carbonate) with the water formed to form acetamide. The reaction conditions required on a 5 millimolar scale (80 bar CO2 pressure, 155 °C reaction temperature, 16 h reaction time) are not yet suitable for an economical industrial synthesis. When CO2 is passed through to strip the water of reaction, a maximum yield of 13% is achieved under optimized conditions.[23]
The direct carbonylation of glycerol with CO2 over highly active cerium(IV) oxide CeO2 catalysts in the presence of the water-binding reagent [2-cyanopyridine and the solvent dimethylformamide DMF at 150 °C, 40 MPa CO2 pressure and 5 h reaction time gives in 10 millimolar batches "... the incredibly high yield of glycerol carbonate..." of up to 78.9%, with the cerium oxide catalyst being regenerated after 5 cycles by calcining at 400 °C.[10]
Characteristics
[edit]Glycerol-1,2-carbonate is a clear colourless viscous liquid with a mild odour,[24] which is completely soluble in water and in polar organic solvents such as e.g. B. alcohols mixes. The compound is in the form of a racemate and can be separated into the enantiomers enzymatically with lipase.[25] The polymers cellulose acetate, nitrocellulose, polyamides and polyacrylonitrile are soluble in glycerol carbonate.[26] The compound is non-flammable, non-toxic and easily biodegradable, it has a low evaporation rate and high moisture retention capacity.[5]
Applications
[edit]Potentially large-volume applications of glycerol-1,2-carbonate would be of enormous interest as the glycerol flood can probably only be managed through caloric utilization and the suitability of glycerol carbonate for the chemical fixation of large amounts of CO2.
At high temperatures, glycerol carbonate decomposes almost exclusively into carbon dioxide and 3-hydroxypropanal HOCH 2 CH 2 CHO, a reactive aldehyde with great potential as a fuel additive to reduce soot formation in internal combustion engines.[27]
Glycerol-1,2-carbonate can be used in particular in cosmetics and personal care products as a protic solvent with a high Permittivity ε=82.7 and humectant.
Because of its similarity to propylene carbonate and because of its non-flammability and high dielectric constants, which allow much higher lithium ion concentrations, hydroxypropylene carbonate is being investigated as an electrolyte in lithium-ion batteries.[9] However, protic solvents with reactive free hydroxy groups are undesirable in battery systems.
The epoxy alcohol glycidol is formed in the decarboxylation of glycerol-1,2-carbonate at temperatures > 175 °C, reduced pressure and in the presence of acidic[28] or basic[29] (e.g. potassium oxide ) catalysts in yields of 75 to 85%
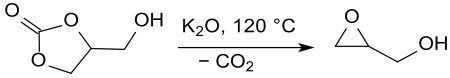
Inexpensive glycidol as a precursor to polyglycerols, glycerol esters, and glycidyl ethers could find wider application in detergents, paints and coatings, and stabilizers for natural oils and for vinyl polymers.
Epichlorohydrin, which is important for the production of epoxy resins and adhesives, can be obtained from glycerol carbonate via 2-chloromethyl-1,3-dioxolane-2-one and its decarboxylation at 80 °C in yields of up to 90%.[22]

Polyglycerol esters are formed by the reaction of long-chain carboxylic acids with glycerol carbonate (and other cyclic carbonates such as ethylene carbonate) in the presence of strong bases and can i.a. as emulsifiers, dispersants, flow improvers and lubricants.[30]

Biodegradable, water-soluble and non-flammable polycarbonate homopolymers are formed when glycerol-1,2-carbonate is heated to 140 °C in the presence of zinc sulfate,
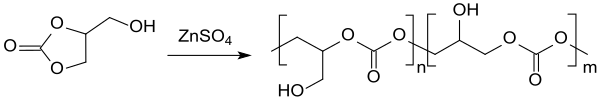
and with excess glycerin, copolymers which might find application as lubricants, detergents, drilling aids, and so forth.[31]
Of particular interest could be the use of bis-glycerol carbonates in non-isocyanate-based polyurethanes (NIPUs), which could replace problematic diisocyanates such as toluene-2,4-diisocyanate TDI and methylene diphenyl diisocyanate MDI.[32][33][34]

Even a partial substitution of diisocyanates in polyurethanes by bis-glycerol carbonates would be a significant outlet for glycerol derivatives and a milestone in the use of monomers from renewable raw materials.[35]
See also
[edit]- Glycerol
- Dioxalin
- Epichlorohydrin
- Nitroglycerin
- Oleochemicals
- Saponification/Soapmaking
- Solketal
- Transesterification
References
[edit]- ^ "Ingredient : HYDROXYPROPYLENE CARBONATE". Retrieved 27 February 2023.
- Glycerol-1,2-carbonate
- ^ a b c d Sigma-Aldrich Co., product no. {{{id}}}.
- ^ a b c Entry from Glycerol-1,2-carbonate from TCI Europe, retrieved on {{{Date}}}
- ^ a b c d "Product Safety Summary for Glycerol carbonate" (PDF). UBE Chemical Europe, S.A. 2016-01-25. Archived from the original (PDF) on 2018-11-01. Retrieved 15 October 2018.
- ^ a b c d "Technical Bulletin JeffsolR Glycerin Carbonate" (PDF). Huntsman Corp. 2008. Archived from the original (PDF) on 22 December 2018. Retrieved 15 October 2018.
- ^ a b K. Lanjekar, V.R. Rathod (2013). "Utilization of glycerol for the production of glycerol carbonate through greener route". Journal of Environmental Chemical Engineering. J. Environ. Chem. Eng. 1 (4): 1231–1236. doi:10.1016/j.jece.2013.09.015.
- ^ "SAFETY DATA SHEET: 4-(Hydroxymethyl)-1,3-dioxolan-2-one" (pdf). Retrieved 27 February 2023.
- ^ Record of 4-Hydroxymethyl-1,3-dioxolan-2-on in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 30 December 2018.
- ^ a b c M.O. Sonnati S. Amigoni, E.P. Taffin de Givenchy, T. Darmanin, O. Chouletb, F. Guittard (2012). Glycerol carbonate as a versatile building block for tomorrow: synthesis, reactivity, properties and applications. Green Chem. Vol. 15. pp. 283–306. doi:10.1039/C2GC36525A.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c J. Liu, Y. Li, J. Zhang, D. He (2016). "Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters". Applied Catalysis A: General. Appl. Catal. A: General. 513: 9–18. doi:10.1016/j.apcata.2015.12.030.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J.I. Garcia, H. Garcia-Marin, E. Pires (2014). Glycerol based solvents: synthesis, properties, and applications. Green Chem. Vol. 16. pp. 1007–1033. doi:10.1039/C3GC41857J. hdl:10261/111769.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b J.R. Ochoa-Gómez, O. Gómez-Jiménez-Aberasturi, C. Ramiréz-López, M. Belsué (2012). "A brief review on industrial alternatives for the manufacturing of glycerol carbonate, a green chemical". Organic Process Research & Development. Org. Process Res. Dev. 16 (3): 389–399. doi:10.1021/op200369v.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ M. North, P. Villuendas, C. Young (2012). "Inter- and intramolecular phosphonium salt cocatalysis in cyclic carbonate synthesis catalysed by a bimetallic aluminium(salen) complex". Tetrahedron Letters. Tetrahedron Lett. 53 (22): 2736–2740. doi:10.1016/j.tetlet.2012.03.090.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Glycerin Market And Industry Analysis, Retail Research, Survey, Global Forecast to 2018". Market Watch. 2018-03-20. Retrieved 2018-10-22.
- ^ L. Zhang, Z. Zhang, C. Wu, Q. Qian, J. Ma, L. Jiang, B. Han (2018). "Microwave assisted synthesis of glycerol carbonate from glycerol and urea". Pure and Applied Chemistry. Pure Appl. Chem. 90 (1): 1–6. doi:10.1515/pac-2017-0303.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J.R. Ochoa-Gómez (2009). "Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization". Applied Catalysis A: General. Appl. Catal. A: General. 366 (2): 315–324. doi:10.1016/j.apcata.2009.07.020.
- ^ F.S.H. Simanjuntak (2011). "CaO-catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate: Isolation and characterization of an active Ca species". Applied Catalysis A: General. Appl. Catal. A: General. 401 (1–2): 220–225. doi:10.1016/j.apcata.2011.05.024.
- ^ a b G. Rokicki, P. Rakoczy, P. Pazuchowski, M. Sobiecki (2005). "Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: glycerol carbonate". Green Chemistry. Green Chem. 7 (7): 529–539. doi:10.1039/B501597A.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J.R. Ochoa-Gómez, O. Gómez-Jiménez-Aberasturi, C. Ramirez-López, B. Maestro-Madurga (2012). "Synthesis of glycerol-1,2-carbonate by transesterification of glycerol with dimethyl carbonate using triethylamine as a facile separable homogeneous catalyst". Green Chemistry. Green Chem. 14 (12): 3368–3376. doi:10.1039/C2GC35992H.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. van Mileghem, W.M. de Borggraeve, I.R. Baxendale (2018). "A robust and scalable continuous flow process for glycerol carbonate". Chemical Engineering & Technology. Chem. Eng. Technol. 41 (10): 2014–2023. doi:10.1002/ceat.201800012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. George, Y. Patel, S.M. Pillai, P. Munshi (2009). "Methanol assisted selective formation of 1,2-glycerol carbonate from glycerol and carbon dioxide using nBu2SnO as a catalyst". Journal of Molecular Catalysis A: Chemical. J. Mol. Catal. A: Chemical. 304 (1–2): 1–7. doi:10.1016/j.molcata.2009.01.010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b A. Dibenedetto, A. Angelini, M. Aresta, J. Ethiraj, C. Fragale, F. Nocito (2011). Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes. Tetrahedron. Vol. 67. pp. 1308–1313. doi:10.1016/j.tet.2010.11.070.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Karolin Schenk (2018). Direct Synthesis of Glycerol Carbonate from Glycerol and Carbon Dioxide by Brønsted Base Catalysis (Dissertation) (Thesis). Aachen: RWTH Aachen.
- ^ "Product Safety Summary for Glycerine carbonate" (PDF). UBE Corp. Europe. 2017-12-14. Retrieved 2018-10-15.
- ^ M. Pallavicini, E. Valoti, L. Villa, O. Piccolo (1994). Lipase-catalyzed resolution of glycerol-2,3-carbonate. J. Org. Chem. Vol. 59. pp. 1751–1754. doi:10.1021/jo00086a026j (inactive 1 November 2024).
{{cite book}}: CS1 maint: DOI inactive as of November 2024 (link) CS1 maint: multiple names: authors list (link) - ^ Mario Pagliaro, Michele Rossi (2010). The Future of Glycerol: New Usages for a Versatile Raw Material, 2nd Edition. Cambridge, UK: RSC Publishing. p. 107. ISBN 978-1-84973-046-4.
- ^ M. Szöri, B.R. Biri, Z. Wang, A.E. Dawood, B. Viskolcz, A. Farooq (2018). "Glycerol carbonate as a fuel additive for sustainable future" (PDF). Sustainable Energy & Fuels. Sustainable Energy Fuels. 2 (10): 2171–2178. doi:10.1039/c8se00207j.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ US 9199950, H.J. Lee, B.S. Ahn, S.D. Lee, J. Jae, J.S. Choi, "Method for producing glycidol by successive catalytic reactions", published 2015-1-12, assigned to Korean Institute of Science and Technology
- ^ EP 3330261, J.R. Ochoa-Goméz, O. Goméz de Miranda Jiménez de Aberastur, N. Blaco Pérez, B. Maestro Madurga, S. Prieto Fernández, "Glycidol synthesis method", published 2018-06-06, assigned to Fundación Tecnalia Research & Innovation
- ^ WO 2008142374, H.S. Bevinakatti, A.G. Waite, J. Frank, "Method of making polyglycerol esters", published 2008-11-27, assigned to Croda International Plc
- ^ US 7928182, T.D. Nguyen, Z. Mouloungui, P. Marechal, "Glycerol polycarbonate, organic compositions containing same and method for obtaining said compositions", published 2011-4-19, assigned to Condat S.A., INRA, INPT
- ^ Guan, Jing; Song, Yihu; Lin, Yu; et al. (1 June 2011). "Progress in Study of Non-Isocyanate Polyurethane". Industrial & Engineering Chemistry Research. 50 (11): 6517–6527. doi:10.1021/ie101995j.
- ^ C. Carré, L. Boet, L. Avérous (2014). "Original biobased nonisocyanate polyurethanes: Solvent- and catalyst-free synthesis, thermal properties and rheological behavior". RSC Adv. RSC Advances. 4 (96): 54016–54025. Bibcode:2014RSCAd...454018C. doi:10.1039/C4RA09794G.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ C. Duval, N. Kébir, R. Jauseau, F. Burel (2016). "Organocatalytic synthesis of novel renewable non-isocyanate polyhydroxy urethanes". Journal of Polymer Science Part A: Polymer Chemistry. Polym. Chem. 54 (6): 758–764. Bibcode:2016JPoSA..54..758D. doi:10.1002/pola.27908.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cornille, Adrien; Auvergne, Rémi; Figovsky, Oleg; et al. (February 2017). "A perspective approach to sustainable routes for non-isocyanate polyurethanes". European Polymer Journal. 87: 535–552. Bibcode:2017EurPJ..87..535C. doi:10.1016/j.eurpolymj.2016.11.027.

