Hexane

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Hexane[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730733 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.435 |
| EC Number |
|
| 1985 | |
| KEGG | |
| MeSH | n-hexane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1208 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14 | |
| Molar mass | 86.178 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Petrolic |
| Density | 0.6548 g mL−1 |
| Melting point | −96 to −94 °C; −141 to −137 °F; 177 to 179 K |
| Boiling point | 68.5 to 69.1 °C; 155.2 to 156.3 °F; 341.6 to 342.2 K |
| 9.5 mg L−1 | |
| log P | 3.764 |
| Vapor pressure | 17.60 kPa (at 20.0 °C) |
Henry's law
constant (kH) |
7.6 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 200 nm |
Refractive index (nD)
|
1.375 |
| Viscosity | 0.3 mPa · s |
| Thermochemistry | |
Heat capacity (C)
|
265.2 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
296.06 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−199.4–−198.0 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−4180–−4140 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H225, H304, H315, H336, H373, H411 | |
| P210, P261, P273, P281, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −26.0 °C (−14.8 °F; 247.2 K) |
| 234.0 °C (453.2 °F; 507.1 K) | |
| Explosive limits | 1.2–7.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
25 g kg−1 (oral, rat) 28710 mg/kg (rat, oral)[3] |
LDLo (lowest published)
|
56137 mg/kg (rat, oral)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 500 ppm (1800 mg/m3)[2] |
REL (Recommended)
|
TWA 50 ppm (180 mg/m3)[2] |
IDLH (Immediate danger)
|
1100 ppm[2] |
| Related compounds | |
Related alkanes
|
|
| Supplementary data page | |
| Hexane (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hexane /ˈhɛkseɪn/ is an alkane of six carbon atoms, with the chemical formula C6H14.
The term may refer to any of the five structural isomers with that formula, or to a mixture of them.[4] In IUPAC nomenclature, however, hexane is the unbranched isomer (n-hexane); the other four isomers are named as methylated derivatives of pentane and butane. IUPAC also uses the term as the root of many compounds with a linear six-carbon backbone, such as 2-methylhexane (C7H16), which is also called "isoheptane".
Hexanes are significant constituents of gasoline. They are all colorless liquids at room temperature, odorless when pure, with boiling points between 50 and 70 °C. They are widely used as cheap, relatively safe, largely unreactive, and easily evaporated non-polar solvents.
Isomers
| Common name | IUPAC name | Text formula | Skeletal formula |
|---|---|---|---|
| normal hexane n-hexane |
hexane | CH3(CH2)4CH3 | |
| isohexane | 2-methylpentane | (CH3)2CH(CH2)2CH3 | 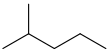
|
| 3-methylpentane | CH3CH2CH(CH3)CH2CH3 | 
| |
| 2,3-dimethylbutane | CH3CH(CH3)CH(CH3)CH3 | 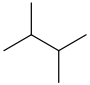
| |
| neohexane | 2,2-dimethylbutane | CH3C(CH3)2CH2CH3 | 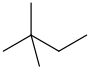
|
Uses
In industry, hexanes are used in the formulation of glues for shoes, leather products, and roofing. They are also used to extract cooking oils (such as canola oil or soy oil) from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. They are commonly used in food based soybean oil extraction in the United States, and are potentially present as contaminants in all soy food products in which the technique is used; the lack of regulation by the FDA of this contaminant is a matter of some controversy.[5][6]
A typical laboratory use of hexanes to extract oil and grease contaminants from water and soil for analysis.[7] Since hexane cannot be easily deprotonated, it is used in the laboratory for reactions that involve very strong bases, such as the preparation of organolithiums. For example, butyllithiums are typically supplied as a hexane solution.
Hexanes are commonly used in chromatography as a non-polar solvent. Higher alkanes present as impurities in hexanes have similar retention times as the solvent, meaning that fractions containing hexane will also contain these impurities. In preparative chromatography, concentration of a large volume of hexanes can result in a sample that is appreciably contaminated by alkanes. This may result in a solid compound being obtained as an oil and the alkanes may interfere with analysis.
In many applications (especially pharmaceutical), the use of n-hexane is being phased out due to its long term toxicity. It is often replaced by n-heptane, which will not form the toxic metabolite hexane-2,5-dione.[8]
Production
Hexanes are chiefly obtained by refining crude oil. The exact composition of the fraction depends largely on the source of the oil (crude or reformed) and the constraints of the refining. The industrial product (usually around 50% by weight of the straight-chain isomer) is the fraction boiling at 65–70 °C.
Physical properties
All alkanes are colorless.[9][10] The boiling points of the various hexanes are somewhat similar and, as for other alkanes, are generally lower for the more branched forms. The melting points are quite different and the trend is not apparent.[11]
| Isomer | M.P. (°C) | B.P. (°C) |
|---|---|---|
| n-hexane | −95.3 | 68.7 |
| 3-methylpentane | −118.0 | 63.3 |
| 2-methylpentane (isohexane) | −153.7 | 60.3 |
| 2,3-dimethylbutane | −128.6 | 58.0 |
| 2,2-dimethylbutane (neohexane) | −99.8 | 49.7 |
Hexane has considerable vapor pressure at room temperature:
| Temperature (°C) | Vapor pressure (mmHg) |
|---|---|
| −40 | 3.36 |
| −30 | 7.12 |
| −20 | 14.01 |
| −10 | 25.91 |
| 0 | 45.37 |
| 10 | 75.74 |
| 20 | 121.26 |
| 25 | 151.28 |
| 30 | 187.11 |
| 40 | 279.42 |
| 50 | 405.31 |
| 60 | 572.76 |
Safety
Acute exposure to n-hexane usually occurs by inhalation, but it may be absorbed orally or through the skin. Minor exposures may occur when people fill their automobile fuel tanks with gasoline. Recent research suggests that certain fungi may be able to produce n-hexane.[12]
The acute toxicity of n-hexane is rather low. However, it has been reported to be the most highly toxic member of the alkanes.[citation needed] When n-hexane is ingested, it causes nausea, vertigo, bronchial irritation, intestinal irritation, and central nervous system effects. It has been reported that ~50 g of n-hexane may be fatal to humans[citation needed]. Furthermore, n-hexane is biotransformed to 2-hexanol and further to 2,5-hexanediol by cytochrome P450 mixed function oxidases by omega oxidation. 2,5-Hexanediol may be further oxidized to 2,5-hexanedione, which is neurotoxic and produces a polyneuropathy.[12]
Products with low viscosity such as hexane and other volatile hydrocarbons (petroleum ether) present an extreme aspiration risk. Even small amounts of a low-viscosity material, once aspirated, can involve a significant portion of the lung and produce a chemical pneumonitis. Hydrocarbon pneumonia is an acute hemorrhagic necrotizing disease that can develop within 24 hours after the aspiration. Pneumonia may require several weeks for complete resolution. Therefore, gastric lavage is not indicated for hydrocarbon ingestion because of the risk of aspiration if the patient vomits around the lavage tube.[13]
n-Hexane is sometimes used as a denaturant for alcohol, and as a cleaning agent in the textile, furniture, and leather industries. It is slowly being replaced with other less toxic solvents.[12]
A peer reviewed study found that inhalation of n-hexane at 5000 ppm for 10 minutes produces marked vertigo; 2500-1000 ppm for 12 hours produces drowsiness, fatigue, loss of appetite, and paresthesia in the distal extremities; 2500–5000 ppm produces muscle weakness, cold pulsation in the extremities, blurred vision, headache and anorexia.[14] The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) for hexane isomers (not n-hexane) of 100 ppm (350 mg/m3) over an 8-hour workday.[15]
Occupational hexane poisoning has occurred with Japanese sandal workers, Italian shoe workers,[16] Taiwan press proofing workers, and others.[17] Analysis of Taiwanese workers has shown occupational exposure to substances including n-hexane.[18] In 2010–2011, Chinese workers manufacturing iPhones were reported to have suffered hexane poisoning.[19][20]
See also
References
- ^ "n-hexane – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 31 December 2011.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0322". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "n-Hexane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "C5 and C6 alkanes". A and B Scott Organic Chemistry. Retrieved 30 October 2007.
- ^ "The Tofurky Company : Our Ingredients". Tofurky.com. Retrieved 17 March 2015.
- ^ Palmer, Brian (26 April 2010). "A study found hexane in soy protein. Should you stop eating veggie burgers?". Slate.com. Retrieved 17 March 2015.
- ^ Use of ozone depleting substances in laboratories. The Nordic Council (2003). ISBN 92-893-0884-2
- ^ Filser JG, Csanády GA, Dietz W, Kessler W, Kreuzer PE, Richter M, Störmer A (1996). "Comparative estimation of the neurotoxic risks of n-hexane and n-heptane in rats and humans based on the formation of the metabolites 2,5-hexanedione and 2,5-heptanedione". Adv Exp Med Biol. 387: 411–427. PMID 8794236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Organic Chemistry-I" (PDF). Nsdl.niscair.res.in. Retrieved 17 February 2014.
- ^ "13. Hydrocarbons | Textbooks". Textbook.s-anand.net. Retrieved 17 February 2014.
- ^ William D. McCain (1990). The properties of petroleum fluids. PennWell. ISBN 0-87814-335-1.
- ^ a b c Clough, Stephen R; Mulholland, Leyna (2005). "Hexane". Encyclopedia of Toxicology. Vol. 2 (2nd ed.). Elsevier. pp. 522–525.
- ^ Gad, Shayne C (2005), "Petroleum Hydrocarbons", Encyclopedia of Toxicology, vol. 3 (2nd ed.), Elsevier, pp. 377–379
- ^ "N-HEXANE". Toxicology data network Hazardous Substances Data Bank. National Library of Medicine.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Hexane isomers (excluding n-Hexane)". www.cdc.gov. Retrieved 3 November 2015.
- ^ Rizzuto, N; De Grandis, D; Di Trapani, G; Pasinato, E (1980). "N-hexane polyneuropathy. An occupational disease of shoemakers". European neurology. 19 (5): 308–15. PMID 6249607.
- ^ n-Hexane, Environmental Health Criteria, World Health Organization, 1991
- ^ Liu, C. H.; Huang, C. Y.; Huang, C. C. (2012). "Occupational Neurotoxic Diseases in Taiwan". Safety and Health at Work. 3 (4): 257–67. doi:10.5491/SHAW.2012.3.4.257. PMC 3521924. PMID 23251841.
- ^ "Workers poisoned while making iPhones – ABC News (Australian Broadcasting Corporation)". Abc.net.au. 26 October 2010. Retrieved 17 March 2015.
- ^ David Barboza (22 February 2011). "Workers Sickened at Apple Supplier in China". The New York Times. Retrieved 17 March 2015.
External links
- International Chemical Safety Card 1262 (2-methylpentane)
- Material Safety Data Sheet for Hexane
- National Pollutant Inventory – n-hexane fact sheet
- Phytochemica l database entry
- Center for Disease Control and Prevention
- Warning from National Safety Council "COMMON CHEMICAL AFFECTS AUTO MECHANICS"
- Australian National Pollutant Inventory (NPI) page
- "EPA does not consider n-hexane classifiable as a human carcinogen." Federal Register / Vol. 66, No. 71 / Thursday, 12 April 2001 / Rules and Regulations

