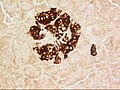
Pancreatic islets
| Pancreatic islets | |
|---|---|
 | |
 A pancreatic islet from a mouse in a typical position, close to a blood vessel; insulin in red, nuclei in blue. | |
| Details | |
| Part of | Pancreas |
| System | Endocrine |
| Identifiers | |
| Latin | insulae pancreaticae |
| MeSH | D007515 |
| TA98 | A05.9.01.019 |
| TA2 | 3128 |
| FMA | 16016 |
| Anatomical terms of microanatomy | |
The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans.[1] The pancreatic islets constitute 1–2% of the pancreas volume and receive 10–15% of its blood flow.[2][3] The pancreatic islets are arranged in density routes throughout the human pancreas, and are important in the metabolism of glucose.[4]
Structure
[edit]There are about 1 million islets distributed throughout the pancreas of a healthy adult human. While islets vary in size, the average diameter is about 0.2 mm.[5]:928 Each islet is separated from the surrounding pancreatic tissue by a thin, fibrous, connective tissue capsule which is continuous with the fibrous connective tissue that is interwoven throughout the rest of the pancreas.[5]:928
Microanatomy
[edit]Hormones produced in the pancreatic islets are secreted directly into the blood flow by (at least) five types of cells. In rat islets, endocrine cell types are distributed as follows:[6]
- Alpha cells producing glucagon (20% of total islet cells)
- Beta cells producing insulin and amylin (≈70%)
- PP cells (gamma cells or F cells) producing pancreatic polypeptide (<5%)
- Delta cells producing somatostatin (<10%)
- Epsilon cells producing ghrelin (<1%)
It has been recognized that the cytoarchitecture of pancreatic islets differs between species.[7][8][9] In particular, while rodent islets are characterized by a predominant proportion of insulin-producing beta cells in the core of the cluster and by scarce alpha, delta and PP cells in the periphery, human islets display alpha and beta cells in close relationship with each other throughout the cluster.[7][9]
The proportion of beta cells in islets varies depending on the species, in humans it is about 40–50%. In addition to endocrine cells, there are stromal cells (fibroblasts), vascular cells (endothelial cells, pericytes), immune cells (granulocytes, lymphocytes, macrophages, dendritic cells,) and neural cells.[10]
A large amount of blood flows through the islets, 5–6 mL/min per 1 g of islet. It is up to 15 times more than in exocrine tissue of the pancreas.[10]
Islets can influence each other through paracrine and autocrine communication, and beta cells are coupled electrically to six to seven other beta cells, but not to other cell types.[11] Pancreatic islets are characterized by rich innervation and vascularization, although there are notable differences between rodent and human islets. Research indicates that the vascular density in human islets is about five times lower than in rodent islets.[12][13] The vascular network within the islets resembles a glomeruli-like structure, consisting of highly fenestrated endothelial cells positioned closely to each endocrine cell.[14] [15] Consequently, the oxygen tension within pancreatic islets is significantly higher than that in the surrounding exocrine tissue.[16]
-
A pancreatic islet, stained.
-
A pancreatic islet, showing alpha cells
-
A pancreatic islet, showing beta cells.
Function
[edit]The paracrine feedback system of the pancreatic islets has the following structure:[17]
- Glucose/Insulin: activates beta cells and inhibits alpha cells.
- Glycogen/Glucagon: activates alpha cells which activates beta cells and delta cells.
- Somatostatin: inhibits alpha cells and beta cells. Also inhibits the secretion of pancreatic polypeptide.[18]
A large number of G protein-coupled receptors (GPCRs) regulate the secretion of insulin, glucagon, and somatostatin from pancreatic islets,[19] and some of these GPCRs are the targets of drugs used to treat type-2 diabetes (ref GLP-1 receptor agonists, DPPIV inhibitors).
-
Mouse islet immunostained for pancreatic polypeptide
-
Mouse islet immunostained for insulin
-
Mouse islet immunostained for glucagon
Electrical activity
[edit]Electrical activity of pancreatic islets has been studied using patch clamp techniques. It has turned out that the behavior of cells in intact islets differs significantly from the behavior of dispersed cells.[20]
Clinical significance
[edit]Diabetes
[edit]The beta cells of the pancreatic islets secrete insulin, and so play a significant role in diabetes. It is thought that they are destroyed by immune assaults.
Because the beta cells in the pancreatic islets are selectively destroyed by an autoimmune process in type 1 diabetes, clinicians and researchers are actively pursuing islet transplantation as a means of restoring physiological beta cell function, which would offer an alternative to a complete pancreas transplant or artificial pancreas.[21][22] Islet transplantation emerged as a viable option for the treatment of insulin requiring diabetes in the early 1970s with steady progress over the following three decades.[23] Clinical trials as of 2008[update] have shown that insulin independence and improved metabolic control can be reproducibly obtained after transplantation of cadaveric donor islets into patients with unstable type 1 diabetes.[22] Alternatively, daily insulin injections are an effective treatment for type 1 diabetes patients who are not candidates for islet transplantation.
People with high body mass index (BMI) are unsuitable pancreatic donors due to greater technical complications during transplantation. However, it is possible to isolate a larger number of islets because of their larger pancreas, and therefore they are more suitable donors of islets.[24]
Islet transplantation only involves the transfer of tissue consisting of beta cells that are necessary as a treatment of this disease. It thus represents an advantage over whole pancreas transplantation, which is more technically demanding and poses a risk of, for example, pancreatitis leading to organ loss.[24] Another advantage is that patients do not require general anesthesia.[25]
Islet transplantation for type 1 diabetes (as of 2008[update]) requires potent immunosuppression to prevent host rejection of donor islets.[26]
The islets are transplanted into a portal vein, which is then implanted in the liver.[24] There is a risk of portal venous branch thrombosis and the low value of islet survival a few minutes after transplantation, because the vascular density at this site is after the surgery several months lower than in endogenous islets. Thus, neovascularization is key to islet survival, that is supported, for example, by VEGF produced by islets and vascular endothelial cells.[10][25] However, intraportal transplantation has some other shortcomings, and so other alternative sites that would provide better microenvironment for islets implantation are being examined.[24] Islet transplant research also focuses on islet encapsulation, CNI-free (calcineurin-inhibitor) immunosuppression, biomarkers of islet damage or islet donor shortage.[27]
An alternative source of beta cells, such insulin-producing cells derived from adult stem cells or progenitor cells would contribute to overcoming the shortage of donor organs for transplantation. The field of regenerative medicine is rapidly evolving and offers great hope for the nearest future. However, type 1 diabetes is the result of the autoimmune destruction of beta cells in the pancreas. Therefore, an effective cure will require a sequential, integrated approach that combines adequate and safe immune interventions with beta cell regenerative approaches.[28] It has also been demonstrated that alpha cells can spontaneously switch fate and transdifferentiate into beta cells in both healthy and diabetic human and mouse pancreatic islets, a possible future source for beta cell regeneration.[29] In fact, it has been found that islet morphology and endocrine differentiation are directly related.[30] Endocrine progenitor cells differentiate by migrating in cohesion and forming bud-like islet precursors, or "peninsulas", in which alpha cells constitute the peninsular outer layer and beta cells form later beneath them. Cryopreservation has shown promise to improve the supply chain of pancreatic islets for better transplantation outcomes. [31]
Additional images
[edit]-
Illustration of dog pancreas. 250x.
Research
[edit]Cannabinoid receptors are found widely expressed in islets of Langerhans, and several studies have investigated specific distribution and mechanisms of CB1 versus CB2 receptors in relation to pancreatic endocrine functions, where they play an important homeostatic role, as endocannabinoids modulate pancreatic β-cells function, proliferation, and survival, as well as insulin production, secretion, and resistance.[32][33][34][35]
See also
[edit]- Betatrophin
- Neuroendocrine tumor
- Pancreatic neuroendocrine tumor
- Adrift Just Off the Islets of Langerhans, a novelette by Harlan Ellison
References
[edit]- ^ Langerhans P (1869). "Beitrage zur mikroscopischen anatomie der bauchspeichel druse". Inaugural-dissertation. Berlin: Gustav Lange.
- ^ Barrett KE, Boitano S, Barman SM, Brooks HL (2009-07-22). Ganong's review of medical physiology (23 ed.). McGraw Hill Medical. p. 316. ISBN 978-0-07-160568-7.
- ^ Functional Anatomy of the Endocrine Pancreas
- ^ Pour PM, Standop J, Batra SK (January 2002). "Are islet cells the gatekeepers of the pancreas?". Pancreatology. 2 (5): 440–448. doi:10.1159/000064718. PMID 12378111. S2CID 37257345.
- ^ a b Feldman M, Friedman LS, Brandt LJ, eds. (2015). Sleisenger & Fordtran's gastrointestinal and liver disease pathophysiology, diagnosis, management (10th ed.). St. Louis, Missouri: Elsevier Health Sciences. ISBN 978-1-4557-4989-8.
- ^ Elayat AA, el-Naggar MM, Tahir M (June 1995). "An immunocytochemical and morphometric study of the rat pancreatic islets". Journal of Anatomy. 186. 186 (Pt 3): 629–637. PMC 1167020. PMID 7559135.
- ^ a b Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. (September 2005). "Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy". The Journal of Histochemistry and Cytochemistry. 53 (9): 1087–1097. doi:10.1369/jhc.5C6684.2005. PMID 15923354.
- ^ Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, et al. (July 2005). "A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations". American Journal of Transplantation. 5 (7): 1635–1645. CiteSeerX 10.1.1.578.5893. doi:10.1111/j.1600-6143.2005.00913.x. PMID 15943621. S2CID 234176.
- ^ a b Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A (February 2006). "The unique cytoarchitecture of human pancreatic islets has implications for islet cell function". Proceedings of the National Academy of Sciences of the United States of America. 103 (7): 2334–2339. Bibcode:2006PNAS..103.2334C. doi:10.1073/pnas.0510790103. PMC 1413730. PMID 16461897.
- ^ a b c Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, et al. (May 2016). "Pancreatic islet blood flow and its measurement". Upsala Journal of Medical Sciences. 121 (2): 81–95. doi:10.3109/03009734.2016.1164769. PMC 4900068. PMID 27124642.
- ^ Kelly C, McClenaghan NH, Flatt PR (2011). "Role of islet structure and cellular interactions in the control of insulin secretion". Islets. 3 (2): 41–47. doi:10.4161/isl.3.2.14805. PMID 21372635.
- ^ Cohrs CM, Chen C, Jahn SR, Stertmann J, Chmelova H, Weitz J, et al. (May 2017). "Vessel Network Architecture of Adult Human Islets Promotes Distinct Cell-Cell Interactions In Situ and Is Altered After Transplantation". Endocrinology. 158 (5): 1373–1385. doi:10.1210/en.2016-1184. PMID 28324008.
- ^ Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, et al. (August 2015). "Human Islets Have Fewer Blood Vessels than Mouse Islets and the Density of Islet Vascular Structures Is Increased in Type 2 Diabetes". The Journal of Histochemistry and Cytochemistry. 63 (8): 637–645. doi:10.1369/0022155415573324. PMID 26216139.
- ^ Bonner-Weir S, Orci L (October 1982). "New perspectives on the microvasculature of the islets of Langerhans in the rat". Diabetes. 31 (10): 883–889. doi:10.2337/diab.31.10.883. PMID 6759221.
- ^ Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A (February 2006). "The unique cytoarchitecture of human pancreatic islets has implications for islet cell function". Proceedings of the National Academy of Sciences of the United States of America. 103 (7): 2334–2339. Bibcode:2006PNAS..103.2334C. doi:10.1073/pnas.0510790103. PMC 1413730. PMID 16461897.
- ^ Carlsson PO, Liss P, Andersson A, Jansson L (July 1998). "Measurements of oxygen tension in native and transplanted rat pancreatic islets". Diabetes. 47 (7): 1027–1032. doi:10.2337/diabetes.47.7.1027. PMID 9648824.
- ^ Wang MB, Bullock J, Boyle JR (2001). Physiology. Hagerstown, MD: Lippincott Williams & Wilkins. p. 391. ISBN 978-0-683-30603-3.
- ^ Marques JM, Nunes R, Florindo H, Ferreira D, Sarmento B (2021). "A demanding path from iPSCs toward pancreatic β- and α-cells". Recent Advances in IPSC-Derived Cell Types. Advances in Stem Cell Biology. 4: 227–256. doi:10.1016/B978-0-12-822230-0.00002-8. ISBN 9780128222300. S2CID 234135648. Retrieved 18 January 2023.
- ^ Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ (September 2013). "An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans". Pharmacology & Therapeutics. 139 (3): 359–391. doi:10.1016/j.pharmthera.2013.05.004. PMID 23694765.
- ^ Pérez-Armendariz M, Roy C, Spray DC, Bennett MV (January 1991). "Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic beta cells". Biophysical Journal. 59 (1): 76–92. Bibcode:1991BpJ....59...76P. doi:10.1016/S0006-3495(91)82200-7. PMC 1281120. PMID 2015391.
- ^ Meloche RM (December 2007). "Transplantation for the treatment of type 1 diabetes". World Journal of Gastroenterology. 13 (47): 6347–6355. doi:10.3748/wjg.13.6347. PMC 4205453. PMID 18081223.
- ^ a b Hogan A, Pileggi A, Ricordi C (January 2008). "Transplantation: current developments and future directions; the future of clinical islet transplantation as a cure for diabetes". Frontiers in Bioscience. 13 (13): 1192–1205. doi:10.2741/2755. PMID 17981623.
- ^ Piemonti L, Pileggi A (2013). "25 Years of the Ricordi Automated Method for Islet Isolation". CellR4-- Repair, Replacement, Regeneration, & Reprogramming. 1 (1): 8–22. PMC 6267808. PMID 30505878.
- ^ a b c d Niclauss N, Meier R, Bédat B, Berishvili E, Berney T (2016-01-27). Stettler C, Christ E, Diem P (eds.). "Beta-Cell Replacement: Pancreas and Islet Cell Transplantation". Endocrine Development. 31. S. Karger AG: 146–162. doi:10.1159/000439412. ISBN 978-3-318-05638-9. PMID 26824893.
- ^ a b Gamble A, Pepper AR, Bruni A, Shapiro AM (March 2018). "The journey of islet cell transplantation and future development". Islets. 10 (2): 80–94. doi:10.1080/19382014.2018.1428511. PMC 5895174. PMID 29394145.
- ^ Chatenoud L (March 2008). "Chemical immunosuppression in islet transplantation--friend or foe?". The New England Journal of Medicine. 358 (11): 1192–1193. doi:10.1056/NEJMcibr0708067. PMID 18337609.
- ^ Chang CA, Lawrence MC, Naziruddin B (October 2017). "Current issues in allogeneic islet transplantation". Current Opinion in Organ Transplantation. 22 (5): 437–443. doi:10.1097/MOT.0000000000000448. PMID 28692442. S2CID 37483032.
- ^ Pileggi A, Cobianchi L, Inverardi L, Ricordi C (October 2006). "Overcoming the challenges now limiting islet transplantation: a sequential, integrated approach". Annals of the New York Academy of Sciences. 1079 (1): 383–398. Bibcode:2006NYASA1079..383P. doi:10.1196/annals.1375.059. PMID 17130583. S2CID 33009393.
- ^ van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dólleman S, et al. (April 2017). "Virgin Beta Cells Persist throughout Life at a Neogenic Niche within Pancreatic Islets". Cell Metabolism. 25 (4): 911–926.e6. doi:10.1016/j.cmet.2017.03.017. PMC 8586897. PMID 28380380.
- ^ Sharon N, Chawla R, Mueller J, Vanderhooft J, Whitehorn LJ, Rosenthal B, et al. (February 2019). "A Peninsular Structure Coordinates Asynchronous Differentiation with Morphogenesis to Generate Pancreatic Islets". Cell. 176 (4) (published 2019): 790–804.e13. doi:10.1016/j.cell.2018.12.003. PMC 6705176. PMID 30661759.
- ^ Zhan L, Rao JS, Sethia N, Slama MQ, Han Z, Tobolt D, et al. (April 2022). "Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation". Nature Medicine. 28 (4): 798–808. doi:10.1038/s41591-022-01718-1. PMC 9018423. PMID 35288694.
- ^ Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, et al. (March 2008). "Presence of functional cannabinoid receptors in human endocrine pancreas". Diabetologia. 51 (3): 476–487. doi:10.1007/s00125-007-0890-y. PMID 18092149.
- ^ Flores LE, Alzugaray ME, Cubilla MA, Raschia MA, Del Zotto HH, Román CL, et al. (October 2013). "Islet cannabinoid receptors: cellular distribution and biological function". Pancreas. 42 (7): 1085–1092. doi:10.1097/MPA.0b013e31828fd32d. PMID 24005231. S2CID 36905885.
- ^ Juan-Picó P, Fuentes E, Bermúdez-Silva FJ, Javier Díaz-Molina F, Ripoll C, Rodríguez de Fonseca F, et al. (February 2006). "Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell". Cell Calcium. 39 (2): 155–162. doi:10.1016/j.ceca.2005.10.005. PMID 16321437.
- ^ Farokhnia M, McDiarmid GR, Newmeyer MN, Munjal V, Abulseoud OA, Huestis MA, et al. (February 2020). "Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study". Translational Psychiatry. 10 (1): 71. doi:10.1038/s41398-020-0756-3. PMC 7031261. PMID 32075958.
External links
[edit]- Pancreas at the Human Protein Atlas