Lead(II) sulfide
| |

| |
| Names | |
|---|---|
| Other names
Plumbous sulfide
Galena | |
| Identifiers | |
| ECHA InfoCard | 100.013.861 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| PbS | |
| Molar mass | 239.30 g/mol |
| Density | 7.60 g/cm3[1] |
| Melting point | 1,118 °C (2,044 °F; 1,391 K) |
| Boiling point | 1,281 °C (2,338 °F; 1,554 K) |
| 2.6×10−11 kg/kg (calculated, at pH=7)[2] 8.6×10−7 kg/kg[3] | |
Solubility product (Ksp)
|
9.04×10−29 |
Refractive index (nD)
|
3.91 |
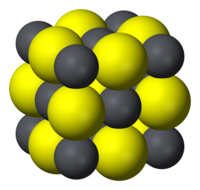
| Structure | |
| Halite (cubic), cF8 | |
| Fm3m, No. 225 | |
| Octahedral (Pb2+) Octahedral (S2−) | |
| Thermochemistry | |
Heat capacity (C)
|
46.02 J/degree mol |
Std molar
entropy (S⦵298) |
–98.7 kJ/mol |
Std enthalpy of
formation (ΔfH⦵298) |
–1.00×102 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Lead(II) oxide Lead selenide Lead telluride |
Other cations
|
Carbon monosulfide Silicon monosulfide Germanium(II) sulfide Tin(II) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lead(II) sulfide (also spelled sulphide) is an inorganic compound with the formula PbTemplate:Sulfur. It finds limited use in electronic devices. PbS, also known as galena, is the principal ore and most important compound of lead.
Formation, basic properties, related materials
Addition of hydrogen sulfide or sulfide salts to a solution of lead ions gives a poorly soluble black product consisting of PbS:
- Pb2+ + H2S → PbS + 2 H+
The equilibrium constant for this reaction is 3×106 M.[4] This reaction, which entails a dramatic color change from colourless or white to black, was once used in qualitative inorganic analysis. The presence of hydrogen sulfide or sulfide ions is still routinely tested using "lead acetate paper."
Like the related materials PbSe and PbTe, PbS is a semiconductor.[5] In fact, lead sulfide was one of the earliest materials to be used as a semiconductor.[6] Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors.
Since PbS is the main ore of lead, much effort has focused on its conversion. A major process involves smelting of PbS followed by reduction of the resulting oxide. Idealized equations for these two steps are:[7]
- 2 PbS + 3 O2 → 2 PbO + 2 SO2
- PbO + C → Pb + CO
The sulfur dioxide is converted to sulfuric acid.
Applications
PbS was once used as a black pigment, but current applications exploit its semiconductor properties, which have long been recognized.[8] PbS is one of the oldest and most common detection element materials in various infrared detectors. As an infrared detector, PbS functions as a photon detector, responding directly to the photons of radiation, as opposed to thermal detectors, which respond to a change in detector element temperature caused by the radiation.
A PbS element can be used to measure radiation in either of two ways: by measuring the tiny photocurrent the photons cause when they hit the PbS material, or by measuring the change in the material's electrical resistance that the photons cause. Measuring the resistance change is the more commonly used method.
At room temperature, PbS is sensitive to radiation at wavelengths between approximately 1 and 2.5 μm. This range corresponds to the shorter wavelengths in the infra-red portion of the spectrum, the so-called short-wavelength infrared (SWIR). Only very hot objects emit radiation in these wavelengths.
Cooling the PbS elements, for example using pressurised or liquified gas or a Peltier element system shifts its sensitivity range to between approximately 2 and 4 μm. Objects that emit radiation in these wavelengths still have to be quite hot—several hundred degrees Celsius—but not as hot as those detectable by uncooled sensors. Other compounds used for this purpose include indium antimonide (InSb) and mercury-cadmium telluride (HgCdTe), which have somewhat better properties for detecting the longer IR wavelengths. The high dielectric constant of PbS leads to relatively slow detectors (compared to silicon, germanium, InSb, or HgCdTe).
Astronomy
Elevations above 2.6 km (1.63 mi) on the planet Venus are coated with a shiny substance. Though the composition of this coat is not entirely certain, one theory is that Venus "snows" crystallized lead sulfide much as Earth snows frozen water. If this is the case, it would be the first time the substance was identified on a foreign planet. Other less likely candidates for Venus' "snow" are bismuth sulfide and tellurium.[9]
Safety
Lead(II) sulfide is non-toxic, unless the lead and sulfur are heated to decomposition and toxic compounds of lead and sulfur oxides are produced (such as in roasting lead ore).[10] Lead sulfide is insoluble and a stable compound in the pH of blood and so it is the least toxic form of lead.[11]
References
- ^ Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. ISBN 0070494398. Retrieved 2009-06-06.
- ^ W. Linke (1965). Solubilities. Inorganic and Metal-Organic Compounds. Vol. 2. Washington, D.C.: American Chemical Society. p. 1318.
- ^ Ronald Eisler (2000). Handbook of Chemical Risk Assessment. CRC Press. ISBN 1566705061.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Vaughan, D. J.; Craig, J. R. (1978). Mineral Chemistry of Metal Sulfides. Cambridge: Cambridge University Press. ISBN 0521214890.
{{cite book}}: CS1 maint: multiple names: authors list (link); - ^ C.Michael Hogan. 2011. Sulfur. Encyclopedia of Earth, eds. A.Jorgensen and C.J.Cleveland, National Council for Science and the environment, Washington DC
- ^ Charles A. Sutherland, Edward F. Milner, Robert C. Kerby, Herbert Teindl, Albert Melin, Hermann M. Bolt (2005). Lead. in Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_193.pub2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Putley, E H; Arthur, J B (1951). "Lead Sulphide – An Intrinsic Semiconductor". Proceedings of the Physical Society Section B. 64: 616. doi:10.1088/0370-1301/64/7/110.
- ^ "'Heavy metal' snow on Venus is lead sulfide". Washington University in St. Louis. Retrieved 2009-07-07.
- ^ Lead sulfide MSDS
- ^ Fritz Bischoff, L. C. Maxwell, Richard D. Evens and Franklin R. Nuzum (1928). "Studies on the Toxicity of Various Lead Compounds Given Intravenously". Journal of Pharmacology and Experimental Therapeutics. 34 (1): 85–109.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

