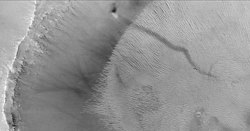
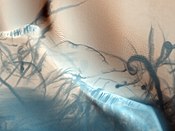
Martian regolith

Martian soil is the fine regolith found on the surface of Mars. Its properties can differ significantly from those of terrestrial soil. The term Martian soil typically refers to the finer fraction of regolith. On Earth, the term "soil" usually includes organic content.[1] In contrast, planetary scientists adopt a functional definition of soil to distinguish it from rocks.[2] Rocks generally refer to 10 cm scale and larger materials (e.g., fragments, breccia, and exposed outcrops) with high thermal inertia, with areal fractions consistent with the Viking Infrared Thermal Mapper (IRTM) data, and immobile under current aeolian conditions.[2] Consequently, rocks classify as grains exceeding the size of cobbles on the Wentworth scale.
This approach enables agreement across Martian remote sensing methods that span the electromagnetic spectrum from gamma to radio waves. ‘‘Soil’’ refers to all other, typically unconsolidated, material including those sufficiently fine-grained to be mobilized by wind.[2] Soil consequently encompasses a variety of regolith components identified at landing sites. Typical examples include: bedform armor, clasts, concretions, drift, dust, rocky fragments, and sand. The functional definition reinforces a recently proposed genetic definition of soil on terrestrial bodies (including asteroids and satellites) as an unconsolidated and chemically weathered surficial layer of fine-grained mineral or organic material exceeding centimeter scale thickness, with or without coarse elements and cemented portions.[1]
Martian dust generally connotes even finer materials than Martian soil, the fraction which is less than 30 micrometres in diameter. Disagreement over the significance of soil's definition arises due to the lack of an integrated concept of soil in the literature. The pragmatic definition "medium for plant growth" has been commonly adopted in the planetary science community but a more complex definition describes soil as "(bio)geochemically/physically altered material at the surface of a planetary body that encompasses surficial extraterrestrial telluric deposits." This definition emphasizes that soil is a body that retains information about its environmental history and that does not need the presence of life to form.
Observations

Mars is covered with vast expanses of sand and dust and its surface is littered with rocks and boulders. The dust is occasionally picked up in vast planet-wide dust storms. Mars dust is very fine and enough remains suspended in the atmosphere to give the sky a reddish hue. The reddish hue is due to rusting iron minerals presumably formed a few billion years ago when Mars was warm and wet, but now that Mars is cold and dry, modern rusting may be due to a superoxide that forms on minerals exposed to ultraviolet rays in sunlight.[5] The sand is believed to move only slowly in the Martian winds due to the very low density of the atmosphere in the present epoch. In the past, liquid water flowing in gullies and river vallies may have shaped the Martian regolith. Mars researchers are studying whether groundwater sapping is shaping the Martian regolith in the present epoch, and whether carbon dioxide hydrates exist on Mars and play a role.

It is believed that large quantities of water and carbon dioxide[citation needed] ices remain frozen within the regolith in the equatorial parts of Mars and on its surface at higher latitudes. Water contents of Martian regolith range from <2% by weight to more than 60%.[7][8] The presence of olivine, which is an easily weatherable primary mineral, has been interpreted to mean that physical rather than chemical weathering processes currently dominate on Mars.[9] High concentrations of ice in soils are thought to be the cause of accelerated soil creep, which forms the rounded "softened terrain" characteristic of the Martian midlatitudes.
In June, 2008, the Phoenix Lander returned data showing Martian soil to be slightly alkaline and containing vital nutrients such as magnesium, sodium, potassium and chloride, all of which are necessary for living organisms to grow. Scientists compared the soil near Mars' north pole to that of backyard gardens on Earth, and concluded that it could be suitable for growth of plants.[10] However, in August, 2008, the Phoenix Lander conducted simple chemistry experiments, mixing water from Earth with Martian soil in an attempt to test its pH, and discovered traces of the salt perchlorate, while also confirming many scientists' theories that the Martian surface was considerably basic, measuring at 8.3. The presence of the perchlorate, if confirmed, would make Martian soil more exotic than previously believed.[11] Further testing is necessary to eliminate the possibility of the perchlorate readings being caused by terrestrial sources, which may have migrated from the spacecraft either into samples or the instrumentation.[12]

While our understanding of Martian soils is extremely rudimentary, their diversity may raise the question of how we might compare them with our Earth-based soils. Applying an Earth-based system is largely debatable but a simple option is to distinguish the (largely) biotic Earth from the abiotic Solar System, and include all non-Earth soils in a new World Reference Base for Soil Resources Reference Group or USDA soil taxonomy Order, which might be tentatively called Astrosols.[13]
On October 17, 2012 (Curiosity rover at "Rocknest"), the first X-ray diffraction analysis of Martian soil was performed. The results revealed the presence of several minerals, including feldspar, pyroxenes and olivine, and suggested that the Martian soil in the sample was similar to the "weathered basaltic soils" of Hawaiian volcanoes.[6] Hawaiian volcanic ash has been used as Martian regolith simulant by researchers since 1998.[14]
In December 2012, scientists working on the Mars Science Laboratory mission announced that an extensive soil analysis of Martian soil performed by the Curiosity rover showed evidence of water molecules, sulphur and chlorine, as well as hints of organic compounds.[3][4][15] However, terrestrial contamination, as the source of the organic compounds, could not be ruled out.
On September 26, 2013, NASA scientists reported the Mars Curiosity rover detected "abundant, easily accessible" water (1.5 to 3 weight percent) in soil samples at the Rocknest region of Aeolis Palus in Gale Crater.[16][17][18][19][20][21] In addition, NASA reported that the Curiosity rover found two principal soil types: a fine-grained mafic type and a locally derived, coarse-grained felsic type.[18][20][22] The mafic type, similar to other martian soils and martian dust, was associated with hydration of the amorphous phases of the soil.[22] Also, perchlorates, the presence of which may make detection of life-related organic molecules difficult, were found at the Curiosity rover landing site (and earlier at the more polar site of the Phoenix lander) suggesting a "global distribution of these salts".[21] NASA also reported that Jake M rock, a rock encountered by Curiosity on the way to Glenelg, was a mugearite and very similar to terrestrial mugearite rocks.[23]
Atmospheric dust

Similarly sized dust will settle from the thinner Martian atmosphere sooner than it would on Earth. For example, the dust suspended by the 2001 global dust storms on Mars only remained in the Martian atmosphere for 0.6 years, while the dust from Mt. Pinatubo took about 2 years to settle.[24] However, under current Martian conditions, the mass movements involved are generally much smaller than on Earth. Even the 2001 global dust storms on Mars moved only the equivalent of a very thin dust layer – about 3 µm thick if deposited with uniform thickness between 58° north and south of the equator.[24] Dust deposition at the two rover sites has proceeded at a rate of about the thickness of a grain every 100 sols.[25]

The difference in the concentration of dust in Earth's atmosphere and that of Mars stems from a key factor. On Earth, dust that leaves atmospheric suspension usually gets aggregated into larger particles through the action of soil moisture or gets suspended in oceanic waters. It helps that most of earth's surface is covered by liquid water. Neither process occurs on Mars, leaving deposited dust available for suspension back into the Martian atmosphere.[26] In fact, the composition of Martian atmospheric dust – very similar to surface dust – as observed by the Mars Global Surveyor Thermal Emission Spectrometer, may be volumetrically dominated by composites of plagioclase feldspar and zeolite[27] which can be mechanically derived from Martian basaltic rocks without chemical alteration. Observations of the Mars Exploration Rovers’ magnetic dust traps suggest that about 45% of the elemental iron in atmospheric dust is maximally (3+) oxidized and that nearly half exists in titanomagnetite,[28] both consistent with mechanical derivation of dust with aqueous alteration limited to just thin films of water.[29] Collectively, these observations support the absence of water-driven dust aggregation processes on Mars. Furthermore, wind activity dominates the surface of Mars at present, and the abundant dune fields of Mars can easily yield particles into atmospheric suspension through effects such as larger grains disaggregating fine particles through collisions.[30]
The Martian atmospheric dust particles are generally 3 µm in diameter.[31] It is important to note that while the atmosphere of Mars is thinner, Mars also has a lower gravitational acceleration, so the size of particles that will remain in suspension cannot be estimated with atmospheric thickness alone. Electrostatic and van der Waals forces acting among fine particles introduce additional complexities to calculations. Rigorous modeling of all relevant variables suggests that 3 µm diameter particles can remain in suspension indefinitely at most wind speeds, while particles as large as 20 µm diameter can enter suspension from rest at surface wind turbulence as low as 2 ms−1 or remain in suspension at 0.8 ms−1.[25]

(Curiosity rover; December 17, 2015).
Gallery
-
Martian sand and boulders photographed by NASA's Mars Exploration Rover Spirit (April 13, 2006).
-
Tracks of the Curiosity rover in the sands of "Hidden Valley" (August 4, 2014).
-
Wheel of the Curiosity rover partially submerged in sand at Hidden Valley (August 6, 2014).
See also
References
- ^ a b Certini, Giacomo; Ugolini, Fiorenzo C. (2013). "An updated, expanded, universal definition of soil". Geoderma. 192: 378–379. doi:10.1016/j.geoderma.2012.07.008.
- ^ a b c Karunatillake, Suniti; Keller, John M.; Squyres, Steven W.; Boynton, William V.; Brückner, Johannes; Janes, Daniel M.; Gasnault, Olivier; Newsom, Horton E. (2007). "Chemical compositions at Mars landing sites subject to Mars Odyssey Gamma Ray Spectrometer constraints". Journal of Geophysical Research. 112. Bibcode:2007JGRE..112.8S90K. doi:10.1029/2006JE002859.
- ^ a b Brown, Dwayne; Webster, Guy; Neal-Jones, Nancy (December 3, 3012). "NASA Mars Rover Fully Analyzes First Martian Soil Samples". NASA. Retrieved December 3, 2012.
{{cite web}}: Check date values in:|date=(help) - ^ a b Chang, Ken (December 3, 2012). "Mars Rover Discovery Revealed". New York Times. Retrieved December 3, 2012.
- ^ Yen, A.S., Kim, S.S., Hecht, M.H., Frant, M.S., Murray, B.; Kim; Hecht; Frant; Murray (2000). "Evidence that the reactivity of the Martian soil is due to superoxide ions". Science. 289 (5486): 1909–12. Bibcode:2000Sci...289.1909Y. doi:10.1126/science.289.5486.1909. PMID 10988066.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Brown, Dwayne (October 30, 2012). "NASA Rover's First Soil Studies Help Fingerprint Martian Minerals". NASA. Retrieved October 31, 2012.
- ^ Mitrofanov, I. et 11 al.; Anfimov; Kozyrev; Litvak; Sanin; Tret'Yakov; Krylov; Shvetsov; Boynton; Shinohara; Hamara; Saunders (2004). "Mineralogy at Gusev crater from the Mössbauer spectrometer on the Spirit rover". Science. 297 (5578): 78–81. Bibcode:2002Sci...297...78M. doi:10.1126/science.1073616. PMID 12040089.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Horneck, G. (2008). "The microbial case for Mars and its implications for human expeditions to Mars". Acta Astronautica. 63 (7–10): 1015–1024. doi:10.1016/j.actaastro.2007.12.002.
- ^ Morris, R.V. et 16 al.; Klingelhöfer; Bernhardt; Schröder; Rodionov; De Souza; Yen; Gellert; Evlanov; Foh; Kankeleit; Gütlich; Ming; Renz; Wdowiak; Squyres; Arvidson (2004). "Mineralogy at Gusev crater from the Mössbauer spectrometer on the Spirit rover". Science. 305 (5685): 833–6. Bibcode:2004Sci...305..833M. doi:10.1126/science.1100020. PMID 15297666.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ "Martian soil 'could support life'". BBC News. June 27, 2008. Retrieved August 7, 2008.
- ^ Chang, Alicia (August 5, 2008). "Scientists: Salt in Mars soil not bad for life". USA Today. Associated Press. Retrieved August 7, 2008.
- ^ "NASA Spacecraft Analyzing Martian Soil Data". JPL. Retrieved August 5, 2008.
- ^ Certini, G, Scalenghe, R, Amundson, R (2009). "A view of extraterrestrial soils". European Journal Soil Science. 60 (6): 1078–1092. doi:10.1111/j.1365-2389.2009.01173.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ L. W. Beegle; G. H. Peters; G. S. Mungas; G. H. Bearman; J. A. Smith; R. C. Anderson (2007). Mojave Martian Simulant: A New Martian Soil Simulant (PDF). Lunar and Planetary Science XXXVIII. Retrieved April 28, 2014.
- ^ Satherley, Dan (December 4, 2012). "'Complex chemistry' found on Mars". 3 News. Retrieved December 4, 2012.
- ^ Lieberman, Josh (September 26, 2013). "Mars Water Found: Curiosity Rover Uncovers 'Abundant, Easily Accessible' Water In Martian Soil". iSciencetimes. Retrieved September 26, 2013.
- ^ Leshin, L. A.; Cabane, M.; Coll, P.; Conrad, P. G.; Archer, P. D.; Atreya, S. K.; Brunner, A. E.; Buch, A.; Eigenbrode, J. L.; Flesch, G. J.; Franz, H. B.; Freissinet, C.; Glavin, D. P.; McAdam, A. C.; Miller, K. E.; Ming, D. W.; Morris, R. V.; Navarro-Gonzalez, R.; Niles, P. B.; Owen, T.; Pepin, R. O.; Squyres, S.; Steele, A.; Stern, J. C.; Summons, R. E.; Sumner, D. Y.; Sutter, B.; Szopa, C. (September 27, 2013). "Volatile, Isotope, and Organic Analysis of Martian Fines with the Mars Curiosity Rover". Science. 341 (6153): 1238937. doi:10.1126/science.1238937. Retrieved September 26, 2013.
- ^ a b Grotzinger, John (September 26, 2013). "Introduction To Special Issue: Analysis of Surface Materials by the Curiosity Mars Rover". Science. 341 (6153): 1475. Bibcode:2013Sci...341.1475G. doi:10.1126/science.1244258. Retrieved September 27, 2013.
- ^ Neal-Jones, Nancy; Zubritsky, Elizabeth; Webster, Guy; Martialay, Mary (September 26, 2013). "Curiosity's SAM Instrument Finds Water and More in Surface Sample". NASA. Retrieved September 27, 2013.
- ^ a b Webster, Guy; Brown, Dwayne (September 26, 2013). "Science Gains From Diverse Landing Area of Curiosity". NASA. Retrieved September 27, 2013.
- ^ a b Chang, Kenneth (October 1, 2013). "Hitting Pay Dirt on Mars". New York Times. Retrieved October 2, 2013.
- ^ a b Meslin, P.-Y.; Forni, O.; Schroder, S.; Cousin, A.; Berger, G.; Clegg, S. M.; Lasue, J.; Maurice, S.; Sautter, V.; Le Mouelic, S.; Wiens, R. C.; Fabre, C.; Goetz, W.; Bish, D.; Mangold, N.; Ehlmann, B.; Lanza, N.; Harri, A.- M.; Anderson, R.; Rampe, E.; McConnochie, T. H.; Pinet, P.; Blaney, D.; Leveille, R.; Archer, D.; Barraclough, B.; Bender, S.; Blake, D.; Blank, J. G.; et al. (September 26, 2013). "Soil Diversity and Hydration as Observed by ChemCam at Gale Crater, Mars". Science. 341 (6153): 1238670. doi:10.1126/science.1238670. Retrieved September 27, 2013.
{{cite journal}}: Explicit use of et al. in:|author2=(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Stolper, E.M.; Baker, M.B.; Newcombe, M.E.; Schmidt, M.E.; Treiman, A.H.; Cousin, A.; Dyar, M.D.; Fisk, M.R.; Gellert, R.; King, P.L.; Leshin, L.; Maurice, S.; McLennan, S.M.; Minitti, M.E.; Perrett, G.; Rowland, S.; Sautter, V.; Wiens, R.C.; MSL ScienceTeam, O.; Bridges, N.; Johnson, J. R.; Cremers, D.; Bell, J. F.; Edgar, L.; Farmer, J.; Godber, A.; Wadhwa, M.; Wellington, D.; McEwan, I.; Newman, C. (2013). "The Petrochemistry of Jake_M: A Martian Mugearite". Science. 341 (6153). AAAS: 1239463. doi:10.1126/science.1239463. Retrieved September 28, 2013.
- ^ a b Cantor, B (2007). "MOC observations of the 2001 Mars planet-encircling dust storm". Icarus. 186: 60–96. Bibcode:2007Icar..186...60C. doi:10.1016/j.icarus.2006.08.019.
- ^ a b Claudin, P; Andreotti, B (2006). "A scaling law for aeolian dunes on Mars, Venus, Earth, and for subaqueous ripples". Earth and Planetary Science Letters. 252: 30–44. arXiv:cond-mat/0603656. Bibcode:2006E&PSL.252...30C. doi:10.1016/j.epsl.2006.09.004.
- ^ Sullivan, R.; Arvidson, R.; Bell, J. F.; Gellert, R.; Golombek, M.; Greeley, R.; Herkenhoff, K.; Johnson, J.; Thompson, S.; Whelley, P.; Wray, J. (2008). "Wind-driven particle mobility on Mars: Insights from Mars Exploration Rover observations at "El Dorado" and surroundings at Gusev Crater". Journal of Geophysical Research. 113: E06S07. Bibcode:2008JGRE..11306S07S. doi:10.1029/2008JE003101.
- ^ Hamilton, Victoria E.; McSween, Harry Y.; Hapke, Bruce (2005). "Mineralogy of Martian atmospheric dust inferred from thermal infrared spectra of aerosols". Journal of Geophysical Research. 110: E12006. Bibcode:2005JGRE..11012006H. doi:10.1029/2005JE002501.
- ^ Goetz et al. (2007), Seventh Mars Conference
- ^ Goetz, W; Bertelsen, P; Binau, Cs; Gunnlaugsson, Hp; Hviid, Sf; Kinch, Km; Madsen, De; Madsen, Mb; Olsen, M; Gellert, R; Klingelhöfer, G; Ming, Dw; Morris, Rv; Rieder, R; Rodionov, Ds; De, Souza, Pa, Jr; Schröder, C; Squyres, Sw; Wdowiak, T; Yen, A; Bertelsen; Binau; Gunnlaugsson; Hviid; Kinch; Madsen; Madsen; Olsen; Gellert; Klingelhöfer; Ming; Morris; Rieder; Rodionov; De Souza; Schröder; Squyres; Wdowiak; Yen (July 2005). "Indication of drier periods on Mars from the chemistry and mineralogy of atmospheric dust". Nature. 436 (7047): 62–5. Bibcode:2005Natur.436...62G. doi:10.1038/nature03807. ISSN 0028-0836. PMID 16001062.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Edgett, Kenneth S. (2002). "Low-albedo surfaces and eolian sediment: Mars Orbiter Camera views of western Arabia Terra craters and wind streaks". Journal of Geophysical Research. 107: 5038. Bibcode:2002JGRE..107.5038E. doi:10.1029/2001JE001587.
- ^ Lemmon, Mt; Wolff, Mj; Smith, Md; Clancy, Rt; Banfield, D; Landis, Ga; Ghosh, A; Smith, Ph; Spanovich, N; Whitney, B; Whelley, P; Greeley, R; Thompson, S; Bell, Jf, 3Rd; Squyres, Sw; Wolff; Smith; Clancy; Banfield; Landis; Ghosh; Smith; Spanovich; Whitney; Whelley; Greeley; Thompson; Bell; Squyres (December 2004). "Atmospheric imaging results from the Mars exploration rovers: Spirit and Opportunity". Science. 306 (5702): 1753–6. Bibcode:2004Sci...306.1753L. doi:10.1126/science.1104474. ISSN 0036-8075. PMID 15576613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link)