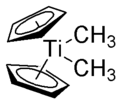
Petasis reagent
| |
| |
| Names | |
|---|---|
| IUPAC name
Bis(η5-cyclopentadienyl)dimethyltitanium
| |
| Other names
Dimethyltitanocene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.204.841 |
| |
| |
| Properties | |
| C12H16Ti | |
| Molar mass | 208.13 g/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant, incompatible with water and oxidizing agents |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The Petasis reagent is an organotitanium compound with the formula Cp2Ti(CH3)2.[1] It is an orange-colored solid.
Preparation and use
It prepared by the salt metathesis reaction of methylmagnesium chloride or methyllithium[2] with titanocene dichloride:[3]
- Cp2TiCl2 + 2 CH3MgCl → Cp2Ti(CH3)2 + 2 MgCl2
The compound is used for transforming carbonyl groups to terminal alkenes. In terms of reactivity, it resembles the Tebbe reagent and Wittig reaction. Unlike the Wittig reaction, the Petasis reagent can react with a wide range of carbonyls, including aldehydes, ketones and esters.[4] The Petasis reagent is also more air stable than the Tebbe reagent, and can be isolated as a pure solid, or used directly as a solution in toluene-THF.
The reaction mechanism is very similar to that of the Tebbe reagent. The active olefinating reagent, Cp2TiCH2, is generated in situ upon heating the solution of Petasis reagent in toluene or THF to 60 °C. With the organic carbonyl, this titanium carbene forms a four membered oxatitanacyclobutane that releases the terminal alkene.[5]
See also
References
- ^ N. A. Petasis and E. I. Bzowej (1990). "Titanium-mediated carbonyl olefinations. 1. Methylenations of carbonyl compounds with dimethyltitanocene". J. Am. Chem. Soc. 112 (17): 6392–6394. doi:10.1021/ja00173a035.
- ^ Claus, K.; Bestian, H. (1962). "Über die Einwirkung von Wasserstoff auf einige metallorganische Verbindungen und Komplexe". Justus Liebigs Ann. Chem. 654: 8. doi:10.1002/jlac.19626540103.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. (2002). "Dimethyltitanocene". Organic Syntheses. 79: 19
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Hartley, R. C.; Li, J.; Main, C. A.; McKiernan, G. J. (2007). "Titanium carbenoid reagents for converting carbonyl groups into alkenes". Tetrahedron. 63 (23): 4825–4864. doi:10.1016/j.tet.2007.03.015.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Meurer, Eduardo Cesar; Santos, Leonardo Silva; Pilli, Ronaldo Aloise; Eberlin, Marcos N. (2003). "Probing the Mechanism of the Petasis Olefination Reaction by Atmospheric Pressure Chemical Ionization Mass and Tandem Mass Spectrometry". Organic Letters. 5 (9): 1391–4. doi:10.1021/ol027439b. PMID 12713281.
