Phenylketonuria: Difference between revisions
→External links: Added link to Canadian PKU Association |
No edit summary |
||
| Line 8: | Line 8: | ||
ICD10 = {{ICD10|E|70|0|e|70}} | |
ICD10 = {{ICD10|E|70|0|e|70}} | |
||
ICD9 = {{ICD9|270.1}} | |
ICD9 = {{ICD9|270.1}} | |
||
ICDO = | |
ICDO = |yo mama was at my house |
||
OMIM = 261600 | |
OMIM = 261600 | |
||
OMIM_mult = {{OMIM2|261630}} | |
OMIM_mult = {{OMIM2|261630}} | |
||
Revision as of 16:16, 7 January 2010
| Phenylketonuria | |
|---|---|
| Specialty | Endocrinology |
Phenylketonuria (PKU) is an autosomal recessive genetic disorder characterized by a deficiency in the hepatic enzyme phenylalanine hydroxylase (PAH).[1]: 541 This enzyme is necessary to metabolize the amino acid phenylalanine ('Phe') to the amino acid tyrosine. When PAH is deficient, phenylalanine accumulates and is converted into phenylpyruvate (also known as phenylketone), which is detected in the urine.[citation needed]
Since its discovery there have been many advances in its treatment. It can now be managed by the patient with little or no side effects, just inconvenience with managing the treatment. If however the condition was left untreated, it can cause problems with brain development, leading to progressive mental retardation, brain damage, and seizures. Historically, PKU has been treated with a low-phenylalanine diet. Research now has proved that diet alone may not be enough to prevent the negative effects of phenylalanine levels. Optimal treatment involves lowering blood Phe levels to a safe range and monitoring diet and cognitive development. Lowering of phenylalanine levels to a safe range may be achieved by combining a low phenylalanine-diet with protein supplements. There is currently no cure for this disease, although some treatments are available with varying success rates. PKU is generally detected through newborn screening and diagnosed by a geneticist. PKU clinics around the world provide integrative care for PKU patients to optimize phe levels, dietary intake and cognitive outcomes.
History
Phenylketonuria was discovered by the Norwegian physician Ivar Asbjørn Følling in 1934[2] when he noticed that hyperphenylalaninemia (HPA) was associated with mental retardation. In Norway, this disorder is known as Følling's disease, named after its discoverer.[3] Dr. Følling was one of the first physicians to apply detailed chemical analysis to the study of disease. His careful analysis of the urine of two affected siblings led him to request many physicians near Oslo to test the urine of other affected patients. This led to the discovery of the same substance that he had found in eight other patients. The substance found was subjected to much more basic and rudimentary chemical analysis (taste). He conducted tests and found reactions that gave rise to benzaldehyde and benzoic acid, which led him to conclude the compound contained a benzene ring. Further testing showed the melting point to be the same as phenylpyruvic acid, which indicated that the substance was in the urine. His careful science inspired many to pursue similar meticulous and painstaking research with other disorders.
Screening and presentation
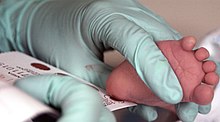
PKU is normally detected using the HPLC test, but some clinics still use the Guthrie test, part of national biochemical screening programs. Most babies in developed countries are screened for PKU soon after birth.[4]
If a child is not screened during the routine Newborn Screening test (typically performed at least 12 hours and generally 24–28 hours after birth, using samples drawn by Neonatal heel prick), the disease may present clinically with seizures, albinism (excessively fair hair and skin), and a "musty odor" to the baby's sweat and urine (due to phenylacetate, one of the ketones produced). In most cases a repeat test should be done at approximately 2 weeks of age to verify the initial test and uncover any phenylketonuria that was initially missed.
Untreated children are normal at birth, but fail to attain early developmental milestones, develop microcephaly, and demonstrate progressive impairment of cerebral function. Hyperactivity, EEG abnormalities and seizures, and severe learning disabilities are major clinical problems later in life. A "musty or mousy" odor of skin, hair, sweat and urine (due to phenylacetate accumulation); and a tendency to hypopigmentation and eczema are also observed.
In contrast, affected children who are detected and treated are less likely to develop neurological problems or have seizures and mental retardation, though such clinical disorders are still possible.
Pathophysiology
Classical PKU is caused by a mutated gene for the enzyme phenylalanine hydroxylase (PAH), which converts the amino acid phenylalanine to other essential compounds in the body. Other non-PAH mutations can also cause PKU. This is an example of genetic heterogeneity.
Classical PKU
The PAH gene is located on chromosome 12 in the bands 12q22-q24.1. More than four hundred disease-causing mutations have been found in the PAH gene. PAH deficiency causes a spectrum of disorders including classic phenylketonuria (PKU) and hyperphenylalaninemia (a less severe accumulation of phenylalanine).[5]
PKU is an autosomal recessive genetic disorder, meaning that each parent must have at least one mutated allele of the gene for PAH, and the child must inherit two mutated alleles, one from each parent. As a result, it is possible for a parent with PKU phenotype to have a child without PKU if the other parent possesses at least one functional allele of the PAH gene; but a child of two parents with PKU will always inherit two mutated alleles, and therefore the disease.
Phenylketonuria can exist in mice, which have been extensively used in experiments into an effective treatment for PKU[6]. The macaque monkey's genome was recently sequenced, and it was found that the gene encoding phenylalanine hydroxylase has the same sequence which in humans would be considered the PKU mutation.[7]
Tetrahydrobiopterin-deficient hyperphenylalaninemia
A rarer form of hyperphenilalaninemia occurs when PAH is normal but there is a defect in the biosynthesis or recycling of the cofactor tetrahydrobiopterin (BH4) by the patient.[8] This cofactor is necessary for proper activity of the enzyme.
Levels of dopamine can be used to distinguish between these two types. Tetrahydrobiopterin is required to convert phenylalanine to tyrosine, but it is also required to convert tyrosine to DOPA (via the enzyme tyrosine hydroxylase), which in turn is converted to dopamine. Low levels of dopamine lead to high levels of prolactin. By contrast, in classical PKU, prolactin levels would be relatively normal. Tetrahydrobiopterin deficiency can be caused by defects in four different genes. These types are known as HPABH4A, HPABH4B, HPABH4C, and HPABH4D.[9]
Metabolic pathways
The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into the amino acid tyrosine. If this reaction does not take place, phenylalanine accumulates and tyrosine is deficient. Excessive phenylalanine can be metabolized into phenylketones through the minor route, a transaminase pathway with glutamate. Metabolites include phenylacetate, phenylpyruvate and phenethylamine[10]. Elevated blood just because phenylalanine and detection of phenylketones in the urine is diagnostic.
Phenylalanine is a large, neutral amino acid (LNAA). LNAAs compete for transport across the blood-brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). Excessive phenylalanine in the blood saturates the transporter. Thus, excessive levels of phenylalanine significantly decrease the levels of other LNAAs in the brain. But since these amino acids are required for protein and neurotransmitter synthesis, phenylalanine accumulation disrupts brain development, leading to mental retardation.[11]
Treatment
If PKU is diagnosed early enough, an affected newborn can grow up with normal brain development, but only by managing and controlling phenylalanine (Phe) levels through diet, or a combination of diet and medication. When phenylalanine can't be metabolized by the body, abnormally high levels accumulate in the blood and are toxic to the brain. When left untreated, complications of PKU include severe mental retardation, brain function abnormalities, microcephaly, mood disorders, irregular motor functioning and abnormal behavior such as ADHD.
All PKU patients must adhere to a special diet low in phenylalanine for at least the first 16 years of their lives. This requires severely restricting or eliminating foods high in phenylalanine, such as meat, chicken, fish, nuts, cheese, legumes and other dairy products. Starchy foods such as potatoes, bread, pasta, and corn must be monitored. Infants may still be breastfed to provide all of the benefits of breastmilk, but the quantity must also be monitored and supplementation for missing nutrients will be required. Many diet foods and diet soft drinks that contain the sweetener aspartame must also be avoided, as aspartame consists of two amino acids: phenylalanine and aspartic acid.
Supplementary infant formulas are used in these patients to provide the amino acids and other necessary nutrients that would otherwise be lacking in a low phenylalanine diet. These can be replaced as the child grows up such as pills, formulas, and specially formulated foods. (Since phenylalanine is necessary for the synthesis of many proteins, it is required for appropriate growth but levels must be strictly controlled in PKU patients). In addition, tyrosine, which is normally derived from phenylalanine, must be supplemented.)
The oral administration of tetrahydrobiopterin (or BH4) (a cofactor for the oxidation of phenylalanine) can reduce blood levels of this amino acid in certain patients.[12][13] The company BioMarin Pharmaceutical has produced a tablet preparation of the compound sapropterin dihydrochloride (Kuvan),which is a form of tetrahydrobiopterin. Kuvan is the first drug that can help BH4-responsive PKU patients (defined among clinicians as about 1/2 of the PKU population) lower Phe levels to recommended ranges.[14] Working closely with a dietitian, some PKU patients who respond to Kuvan may also be able to increase the amount of natural protein they can eat.[15] After extensive clinical trials, Kuvan has been approved by the FDA for use in PKU therapy. Researchers and clinicians working with PKU are finding Kuvan a safe and effective addition to dietary treatment and beneficial to patients with PKU.[16][17]
There are a number of other therapies currently under investigation, including gene therapy, large neutral amino acids, and enzyme substitution therapy with phenylalanine ammonia lyase (PAL). Previously, PKU-affected people were allowed to go off diet after approximately 8, then 18 years of age. Today most physicians recommend that PKU patients need to manage their Phe levels throughout life.
Maternal phenylketonuria

For women affected with PKU, it is essential for the health of their child to maintain low phenylalanine levels before and during pregnancy.[18] Though the developing fetus may only be a carrier of the PKU gene, the intrauterine environment can have very high levels of phenylalanine, which can cross the placenta. The result is that the child may develop congenital heart disease, growth retardation, microcephaly and mental retardation.[19] PKU-affected women themselves are not at risk from additional complications during pregnancy.
In most countries, women with PKU who wish to have children are advised to lower their blood phenylalanine levels (typically to between 2 and 6 micromol/deciliter) before they become pregnant and carefully control their phenylalanine levels throughout the pregnancy. This is achieved by performing regular blood tests and adhering very strictly to a diet, generally monitored on a day-to-day basis by a specialist metabolic dietitian. In many cases, as the fetus' liver begins to develop and produce PAH normally, the mother's blood phenylalanine levels will drop, requiring an increased phenylalanine intake to remain within the safe range of 2-6 micromol/dL. The mother's daily phenylalanine intake may double or even triple by the end of the pregnancy as a result. When maternal blood phenylalanine levels fall below 2 micromol/dL, anecdotal reports indicate that the mothers may suffer adverse effects including headaches, nausea, hair loss, and general malaise. When low phenylalanine levels are maintained for the duration of pregnancy there are no elevated levels of risk of birth defects compared with a baby born to a non-PKU mother.[20] Babies with PKU may drink breast milk, while also taking their special metabolic formula. Some research has indicated that an exclusive diet of breast milk for PKU babies may alter the effects of the deficiency, though during breastfeeding the mother must maintain a strict diet to keep their phenylalanine levels low. More research is needed.
Incidence
The incidence of PKU is about 1 in 15,000 births, but the incidence varies widely in different human populations from 1 in 4,500 births among the population of Ireland[21] to 1 in 13,000 births in Norway[22] to fewer than one in 100,000 births among the population of Finland.[23] Turkey, at 1 in 2600, has the highest incidence rate in the world. The illness is also more common in Italy and China, as well as in Yemeni populations[24].
In relationships
It was discovered in 2007 that those with this disorder will discharge a concentrated amount of phenylalanine in breast milk and semen.[citation needed] If these bodily fluids are transferred between two individual phenylketonurics, there is a significant health risk to the receiving partner. The risk, however, has been determined to be statistically insignificant (for each exchange of bodily fluid, the risk is 1 in 15,000 squared, or, 1 in 225,000,000.) Since there have been no reported cases, the risk is theoretical. It was noted, however, that since the rise of the Internet, people coping with this disorder have sought each other out, so the increased social interaction may become a cause for concern.[citation needed]
See also
References
- ^ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Folling, A. (1934). "Ueber Ausscheidung von Phenylbrenztraubensaeure in den Harn als Stoffwechselanomalie in Verbindung mit Imbezillitaet". Ztschr. Physiol. Chem. 227: 169–176.
- ^ Centerwall, S. A. & Centerwall, W. R. (2000). "The discovery of phenylketonuria: the story of a young couple, two affected children, and a scientist". Pediatrics. 105 (1 Pt 1): 89–103. doi:10.1542/peds.105.1.89. PMID 10617710.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mayo Clinic Staff (2007-12-20). "Phenylketonuria (PKU)". Mayo Clinic. Retrieved 2008-03-13.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ http://www.genenames.org Phenylalanine hydroxylase (PAH) gene summary, retrieved September 8, 2006
- ^ Oh, H. J., Park, E. S., Kang, S., Jo, I., Jung, S. C. (2004). "Long-Term Enzymatic and Phenotypic Correction in the Phenylketonuria Mouse Model by Adeno-Associated Virus Vector-Mediated Gene Transfer". Pediatric Research. 56: 278–284. doi:10.1203/01.PDR.0000132837.29067.0E. PMID 15181195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gibbs, Richard A. (2007). "Evolutionary and Biomedical Insights from the Rhesus Macaque Genome". Science. 316 (5822): 222–234. doi:10.1126/science.1139247. PMID 17431167. Retrieved 2008-02-26.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Surtees, R., Blau, N. (2000). "The neurochemistry of phenylketonuria". European Journal of Pediatrics. 169: S109–13. doi:10.1007/PL00014370. PMID 11043156.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Online Mendelian Inheritance in Man (OMIM): 261640
- ^ Michals, K., Matalon, R. (1985). "Phenylalanine metabolites, attention span and hyperactivity". American Journal of Clinical Nutrition. 42(2): 361–365. PMID 4025205.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pietz, J., Kreis, R., Rupp, A., Mayatepek, E., Rating, D., Boesch, C., Bremer, H. J. (1999). "Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria". Journal of Clinical Investigation. 103: 1169–1178. doi:10.1172/JCI5017. PMID 10207169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burton, BK (2008). "Fresh from the Pipeline: Sapropterin". Nature Reviews Drug Discovery. 7: 199–200. doi:10.1038/nrd2540.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Michals-Matalon K (2008). "Sapropterin dihydrochloride, 6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, in the treatment of phenylketonuria". Expert Opin Investig Drugs. 17 (2): 245–51. doi:10.1517/13543784.17.2.245. PMID 18230057.
- ^ Burton BK, Grange DK, Milanowski A, Vockley G, Feillet F, Crombez EA; et al. (2007). "The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study". Journal of Inherited Metabolic Disorders. 30: 700–707. doi:10.1007/s10545-007-0605-z. PMID 17846916.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Levy H, Burton B, Cederbaum S; et al. (2007). "Recommendations for evaluation of responsiveness to tetrahydrobiopterin (BH(4)) in phenylketonuria and its use in treatment". Mol Genet Metab. 92 (4): 287–291. doi:10.1016/j.ymgme.2007.09.017. PMID 18036498.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK; et al. (2007). "Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study". Lancet. 370: 504–510. doi:10.1016/S0140-6736(07)61234-3. PMID 17693179.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lee P, Treacy E, Crombez E; et al. (2008). "Safety and efficacy of 22 weeks of treatment with sapropterin dihydrochloride in patients with phenylketonuria". Am J Med Genet. 146A (22): 2851–2859. doi:10.1002/ajmg.a.32562. PMID 18932221.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Lee, P.J., Ridout, D., Walker, J.H., Cockburn, F., (2005). "Maternal phenylketonuria: report from the United Kingdom Registry 1978–97". Archives of Disease in Childhood. 90: 143–146. doi:10.1136/adc.2003.037762. PMID 15665165.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link). - ^ Rouse, B., Azen, B., Koch, R., Matalon, R., Hanley, W., de la Cruz, F., Trefz, F., Friedman, E., Shifrin, H. (1997). "Maternal phenylketonuria collaborative study (MPKUCS) offspring: Facial anomalies, malformations, and early neurological sequelae". American Journal of Medical Genetics. 69 (1): 89–95. doi:10.1002/(SICI)1096-8628(19970303)69:1<89::AID-AJMG17>3.0.CO;2-K. PMID 9066890.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ lsuhsc.edu Genetics and Louisiana Families
- ^ DiLella, A. G., Kwok, S. C. M., Ledley, F. D., Marvit, J., Woo, S. L. C. (1986). "Molecular structure and polymorphic map of the human phenylalanine hydroxylase gene". Biochemistry. 25: 743–749. doi:10.1021/bi00352a001. PMID 3008810.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ www.rikshospitalet.no
- ^ Guldberg, P., Henriksen, K. F., Sipila, I., Guttler, F., de la Chapelle, A. (1995). "Phenylketonuria in a low incidence population: molecular characterization of mutations in Finland". J. Med. Genet. 32: 976–978. doi:10.1136/jmg.32.12.976. PMID 8825928.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://emedicine.medscape.com/article/947781-overview
