Sodium stibogluconate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.909 |
| Chemical and physical data | |
| Formula | C12H38Na3O26Sb2 |
| Molar mass | 910.9 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sodium stibogluconate is a medicine used to treat leishmaniasis and is only available for administration by injection. It belongs to the class of drugs known as the pentavalent antimonials because they contain antimony in its oxidation state of 5. Sodium stibogluconate is marketed under the name Pentostam. In parts of the world resistance has limited the utility of sodium stibogluconate,[1] in which cases amphotericin or miltefosine is used instead.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[2]
Side effects
Sodium stibogluconate is exceedingly phlebotoxic. One of the practical problems is that after a few doses it can become exceedingly difficult to find a vein in which to inject the drug. The insertion of a PICC does not prevent the problem and can instead exacerbate it: the entire vein along the course of the PICC line can become inflamed and thrombose. Large doses of sodium stibogluconate are often administered as dilute solutions.
Pancreatitis is a common problem and the serum amylase or lipase should be monitored twice weekly; there is no need to stop treatment if the amylase remains less than four times the upper limit of normal; if the amylase rises above the cut-off then treatment should be interrupted until the amylase falls to less than twice the upper limit of normal, whereupon treatment can be resumed. Cardiac conduction disturbances are less common, but ECG monitoring while the medicine is injected is advisable and changes quickly reverse after the drug is stopped or the infusion rate is decreased.
The drug can be given intramuscularly but is exceedingly painful when given by this route. It can also be given intralesionally when treating cutaneous leishmaniasis (i.e., injected directly into the area of infected skin) and again, this is exceedingly painful and does not give results superior to intravenous administration.
Sodium stibogluconate can also cause a reduced appetite, metallic taste in mouth, nausea, vomiting, diarrhoea, headache, tiredness, joint pains, muscle aches, dizziness, and anaphylaxis.
Dosing
Sodium stibogluconate is available in the United Kingdom as Pentostam, where it is manufactured by GlaxoSmithKline. It is available in the United States on a named-patient basis from the Centers for Disease Control and Prevention (CDC).
The dose of sodium stibogluconate is by slow intravenous infusion (at least five minutes with cardiac monitoring). The injection are stopped if there is coughing or central chest pain. The chemotherapeutic index was established by Leonard Goodwin during the Second World War when a treatment was urgently required for Allied troops during the invasion of Sicily.[3]
The duration of treatment is usually 10 to 21 days and depends on the species of Leishmania and the type of infection (cutaneous or visceral).
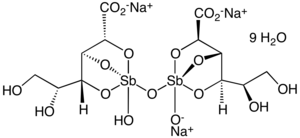
Chemical structure
The chemical structure of sodium stibogluconate is somewhat ambiguous, and the structure shown above is idealized. Its solutions may contain multiple antimony compounds, although this heterogeneity may be unimportant. It has been speculated that the active species contains only a single antimony centre.[4]
Pharmacokinetics
Although antimony itself is a cause of heavy metal poisoning, stibogluconate does not appear to accumulate in the body and is excreted by the kidneys.[5]
References
- ^ Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, Rosen BP (1996). "Trypanothione Overproduction and Resistance to Antimonials and Arsenicals in Leishmania" (pdf). PNAS. 93 (19): 10383–10387. doi:10.1073/pnas.93.19.10383. PMC 38393. PMID 8816809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ "Leonard Goodwin - Telegraph". The Daily Telegraph. 14 January 2009. Retrieved 2009-01-18.
- ^ Frézard F, Demicheli C, Ribeiro PR (2009). "Pentavalent Antimonials: New Perspectives for Old Drugs" (pdf). Molecules. 14 (7): 2317–2336. doi:10.3390/molecules14072317. PMID 19633606.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Rees PH, Keating MI, Kager PA, Hockmeyer WT (1980). "Renal clearance of pentavalent antimony (sodium stibogluconate)". Lancet. 2 (8188): 226–9. doi:10.1016/s0140-6736(80)90120-8. PMID 6105394.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- British National Formulary
- The Merck Index, 12th Edition. 742
