Staphylococcus epidermidis
| Staphylococcus epidermidis | |
|---|---|
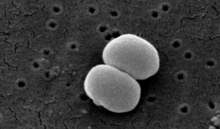
| |
| Scanning electron image of S. epidermidis. | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | S. epidermidis
|
| Binomial name | |
| Staphylococcus epidermidis (Winslow & Winslow 1908)
Evans 1916 | |
| Synonyms | |
|
Staphylococcus albus Rosenbach 1884 | |
Staphylococcus epidermidis is a Gram-positive bacterium, and one of over 40 species belonging to the genus Staphylococcus.[1] It is part of the normal human flora, typically the skin flora, and less commonly the mucosal flora.[2] It is a facultative anaerobic bacteria. Although S. epidermidis is not usually pathogenic, patients with compromised immune systems are at risk of developing infection. These infections are generally hospital-acquired.[3] S. epidermidis is a particular concern for people with catheters or other surgical implants because it is known to form biofilms that grow on these devices.[4] Being part of the normal skin flora, S. epidermidis is a frequent contaminant of specimens sent to the diagnostic laboratory.[5]

Etymology
'Staphylococcus' - bunch of grape-like berries, 'epidermidis' - of the epidermis [6]
Discovery
Friedrich Julius Rosenbach distinguished S. epidermidis from S. aureus in 1884, initially naming S. epidermidis as S. albus.[7] He chose aureus and albus since the bacteria formed yellow and white colonies, respectively.
Cellular morphology and biochemistry
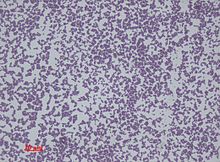
S. epidermidis is a very hardy microorganism, consisting of nonmotile, Gram-positive cocci, arranged in grape-like clusters. It forms white, raised, cohesive colonies about 1–2 mm in diameter after overnight incubation, and is not hemolytic on blood agar.[4] It is a catalase-positive,[8] coagulase-negative, facultative anaerobe that can grow by aerobic respiration or by fermentation. Some strains may not ferment.[9]
Biochemical tests indicate this microorganism also carries out a weakly positive reaction to the nitrate reductase test. It is positive for urease production, is oxidase negative, and can use glucose, sucrose, and lactose to form acid products. In the presence of lactose, it will also produce gas. S. epidermidis does not possess the gelatinase enzyme, so it cannot hydrolyze gelatin.[citation needed] It is sensitive to novobiocin, providing an important test to distinguish it from Staphylococcus saprophyticus, which is coagulase-negative, as well, but novobiocin-resistant.[3]
Similar to those of S. aureus, the cell walls of S. epidermidis have a transferrin-binding protein that helps the organism obtain iron from transferrin. The tetramers of a surface exposed protein, glyceraldehyde-3-phosphate dehydrogenase, are believed to bind to transferrin and remove its iron. Subsequent steps include iron being transferred to surface lipoproteins, then to transport proteins which carry the iron into the cell.[4]
Virulence and antibiotic resistance
The ability to form biofilms on plastic devices is a major virulence factor for S. epidermidis. One probable cause is surface proteins that bind blood and extracellular matrix proteins. It produces an extracellular material known as polysaccharide intercellular adhesin (PIA), which is made up of sulfated polysaccharides. It allows other bacteria to bind to the already existing biofilm, creating a multilayer biofilm. Such biofilms decrease the metabolic activity of bacteria within them. This decreased metabolism, in combination with impaired diffusion of antibiotics, makes it difficult for antibiotics to effectively clear this type of infection.[4] S. epidermidis strains are often resistant to antibiotics, including rifamycin, fluoroquinolones, gentamicin, tetracycline, clindamycin, and sulfonamides.[10] Methicillin resistance is particularly widespread, with 75-90% of hospital isolates resistance to methicilin.[10] Resistant organisms are most commonly found in the intestine, but organisms living freely on the skin can also become resistant due to routine exposure to antibiotics secreted in sweat.
Disease

As mentioned above, S. epidermidis causes biofilms to grow on plastic devices placed within the body.[11] This occurs most commonly on intravenous catheters and on medical prostheses.[12] Infection can also occur in dialysis patients or anyone with an implanted plastic device that may have been contaminated. It also causes endocarditis, most often in patients with defective heart valves. In some other cases, sepsis can occur in hospital patients. [citation needed]
Antibiotics are largely ineffective in clearing biofilms. The most common treatment for these infections is to remove or replace the infected implant, though in all cases, prevention is ideal. The drug of choice is often vancomycin, to which rifampin or aminoglycoside can be added.[citation needed] Hand washing has been shown to reduce the spread of infection.
Preliminary research also indicates S. epidermidis is universally found inside affected acne vulgaris pores, where Propionibacterium acnes is normally the sole resident.[13]
Identification
The normal practice of detecting S. epidermidis is by using appearance of colonies on selective media, bacterial morphology by light microscopy, catalase and slide coagulase testing. On the Baird-Parker agar with egg yolk supplement, colonies appear small and black. Increasingly, techniques such as quantitative PCR are being employed for the rapid detection and identification of Staphylococcus strains.[14][15] Normally, sensitivity to desferrioxamine can also be used to distinguish it from most other staphylococci, except in the case of Staphylococcus hominis, which is also sensitive.[16] In this case, the production of acid from trehalose by S. hominis can be used to tell the two species apart.
See also
Notes and references
- ^ Schleifer, K. H.; Kloos, W. E. (1975). "Isolation and Characterization of Staphylococci from Human Skin I. Amended Descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and Descriptions of Three New Species Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus". International Journal of Systematic Bacteriology. 25 (1): 50–61. doi:10.1099/00207713-25-1-50. ISSN 0020-7713.
- ^ Fey, P. D.; Olson, M. E. (2010). "Current concepts in biofilm formation of Staphylococcus epidermidis". Future Microbiology. 5 (6): 917–933. doi:10.2217/fmb.10.56. PMC 2903046.
- ^ a b Levinson, W. (2010). Review of Medical Microbiology and Immunology (11th ed.). pp. 94–99.
- ^ a b c d Salyers, Abigail A.; Whitt, Dixie D. (2002). Bacterial Pathogenesis: A Molecular Approach, 2nd ed. Washington, D.C.: ASM Press. ISBN 1-55581-171-X.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Queck SY, Otto M (2008). "Staphylococcus epidermidis and other Coagulase-Negative Staphylococci". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 978-1-904455-29-5.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ http://www.vetbact.org/vetbact/?artid=205
- ^ Friedrich Julius Rosenbach at Who Named It?
- ^ "Todar's Online Textbook of Bacteriology: Staphylococcus aureus and Staphylococcal Disease". Kenneth Todar, PhD. Retrieved Dec 7, 2013.
- ^ "Bacteria Genomes - STAPHYLOCOCCUS EPIDERMIDIS". Karyn's Genomes. EMBL-EBI. Retrieved December 23, 2011.
- ^ a b Otto M (August 2010). "Staphylococcus epidermidis - the "accidental" pathogen". Nature Reviews Microbiology. 7 (8): 555–567. doi:10.1038/nrmicro2182. PMC 2807625.
- ^ Otto M (2009), "Staphylococcus epidermidis — the 'accidental' pathogen", Nature Reviews Microbiology, 7 (8): 555–567, doi:10.1038/nrmicro2182, PMC 2807625, PMID 19609257
- ^ Hedin G (1993), "Staphylococcus epidermidis — hospital epidemiology and the detection of methicillin resistance", Scandinavian Journal of Infectious Diseases Supplementum, 90, Oslo Norway: Scandinavian University Press: 1–59, PMID 8303217
- ^ Bek-Thomson, M.; et al. (2008). "Acne is Not Associated with Yet-Uncultured Bacteria". Journal of Clinical Microbiology. 46 (10): 3355–3360. doi:10.1128/JCM.00799-08. PMC 2566126. PMID 18716234.
- ^ Francois P, Schrenzel J (2008). "Rapid Diagnosis and Typing of Staphylococcus aureus". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 978-1-904455-29-5.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Mackay IM (editor). (2007). Real-Time PCR in Microbiology: From Diagnosis to Characterization. Caister Academic Press. ISBN 978-1-904455-18-9.
{{cite book}}:|author=has generic name (help) - ^ Antunes, Ana Lúcia Souza; Secchi, Carina; Reiter, Keli Cristine; Perez, Leandro Reus Rodrigues; Freitas, Ana Lúcia Peixoto De; D'azevedo, Pedro Alves (2008-01-01). "Feasible identification of Staphylococcus epidermidis using desferrioxamine and fosfomycin disks". APMIS. 116 (1): 16–20. doi:10.1111/j.1600-0463.2008.00796.x. ISSN 1600-0463.
External links
- Type strain of Staphylococcus epidermidis at BacDive - the Bacterial Diversity Metadatabase
- Teruaki Nakatsuji et al.: A commensal strain of Staphylococcus epidermidis protects against skin neoplasia, in: Science Advances; 28th of Feb., 2018; Vol. 4, No. 2, DOI:10.1126/sciadv.aao4502
