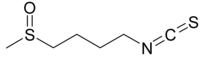
Sulforaphane
| |
| |
| |
| Names | |
|---|---|
| IUPAC name
1-Isothiocyanato-4-methylsulfinylbutane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H11NOS2 | |
| Molar mass | 177.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulforaphane is a molecule within the isothiocyanate group of organosulfur compounds. It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts or cabbages. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing), which allows the two compounds to mix and react. Young sprouts of broccoli and cauliflower are particularly rich in glucoraphanin.
 glucoraphanin, glucosinolate precursor to sulforaphane |
Occurrence and isolation
Sulforaphane was identified in broccoli sprouts, which, of the cruciferous vegetables, have the highest concentration of sulforaphane.[1] It is also found in Brussels sprouts, cabbage, cauliflower, bok choy, kale, collards, Chinese broccoli, broccoli raab, kohlrabi, mustard, turnip, radish, arugula, and watercress.
Research
Sulforaphane has attracted recent research attention for its antioxidant properties, which may persist for hours after ingestion.[2]
Basic research on sulforaphane indicates potential for effects on mechanisms of human disease, including neurodegenerative disorders and cancer; however, results to date are contradictory, requiring clarification by future studies.[3][4] In animal models sulforaphane appears to have a protective effect against diabetes-related kidney damage.[2]
Sulforaphane may have a neuroprotective effect that can aid recovery from spinal cord injury, though the effect is not as strong as that of interleukin-10.[5]
See also
References
- ^ Zhang Y, Talalay P, Cho CG, Posner GH (March 1992). "A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2399–2403. doi:10.1073/pnas.89.6.2399. PMC 48665. PMID 1549603.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Dadras F, Khoshjou F (September 2013). "NF-E2-related factor 2 and its role in diabetic nephropathy". Iran J Kidney Dis (Review). 7 (5): 346–51. PMID 24072144.
- ^ Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P (2013). "Sulforaphane as a potential protective phytochemical against neurodegenerative diseases". Oxid Med Cell Longev (Review). 2013: 415078. doi:10.1155/2013/415078. PMC 3745957. PMID 23983898.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Grabacka MM, Gawin M, Pierzchalska M (2014). "Phytochemical modulators of mitochondria: the search for chemopreventive agents and supportive therapeutics". Pharmaceuticals (Basel) (Review). 7 (9): 913–42. doi:10.3390/ph7090913. PMC 4190497. PMID 25192192.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Koushki D, Latifi S, Javidan AN, Matin M (June 2014). "Efficacy of some non-conventional herbal medications (sulforaphane, tanshinone IIA, and tetramethylpyrazine) in inducing neuroprotection in comparison with interleukin-10 after spinal cord injury: A meta-analysis". J Spinal Cord Med (Meta-analysis). doi:10.1179/2045772314Y.0000000215. PMID 24969510.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
